-
月季(Rosa hybrida)切花作为世界四大切花之首,年贸易额占世界切花总贸易额的32%[1].然而月季切花不耐贮运,发达国家采后损耗通常在20%,我国往往超过30%,有时甚至高达50%,造成巨大的经济损失[2].主要表现为月季花朵的不正常开放,如僵花、僵蕾,提前衰老等[3-5].因此,解析月季切花采后花朵衰老机制始终是国际热点.
目前,影响月季切花衰老的主要因素分为两大类,一类是外部因素,如光照、温度、机械损伤等;另一类是内部因素,如水分平衡、大分子物质代谢、生物膜透性变化以及内源植物激素变化等.月季瓶插期间,由于次生代谢产物积累以及病菌侵染,往往造成木质部导管堵塞,减少水分的吸收与运输,破坏水分平衡,最终加速花朵衰老[6].失水胁迫会显著提高月季萼片中乙烯生物合成酶基因RhACS1与RhACS2表达量,诱导乙烯大量合成[7].此外,在蛋白水平,月季通过精准地调控雌蕊中RhMPK6-RhACS1级联反应,进而诱导乙烯大量产生[8].同时,膜脂过氧化也是引起月季切花衰老的因素之一[9].细胞膜透性增加会加剧水分丧失,引起月季切花提前衰老[10].相反,抗坏血酸和β-胡萝卜素处理能够有效抑制月季切花的衰老进程并延长切花瓶插寿命,可能与提高细胞膜保护酶活性有关[11].
此外,月季花朵的衰老受植物激素调控.乙烯处理引起月季花朵加速开放,花朵开放期(2至4级)较对照的5 d缩短为3.0~3.4 d;然而,乙烯抑制剂1-MCP处理能显著延长花朵开放期,达到7.6~8.1 d[12].在分子水平,一个月季HD-Zip Ⅰ家族RhHB1转录因子介导了GAs、ABA和乙烯3种激素在月季衰老进程中的拮抗调节[13].总体来说,乙烯与ABA促进月季花朵的衰老,而细胞分裂素和赤霉素发挥延缓功能[14].
通常,当植物遭受病菌侵染时会特异诱导某些基因的表达,其中包括病程相关基因(PR).基于蛋白结构和功能特性,将PR蛋白划分为17不同的家族.其中,PR-10蛋白家族包括100多个成员,其开放阅读框(ORF)全长通常包含456至489 bp,编码151~162个氨基酸,具有一个保守的P-loop基序(GxGGxGxxK),定位于细胞质中[15].在前期工作中,我们证明了一个受月季花朵衰老显著诱导的PR10蛋白家族基因RhPR10.1,通过调控花朵内源细胞分裂素浓度,进而拮抗乙烯诱导的月季花朵衰老[16].然而,月季PR-10蛋白家族的其他成员是否也参与了花朵衰老调控仍然未知.本研究从月季花瓣中分离了另一个PR-10蛋白家族基因RhPR10.2,并对其蛋白特性及生物学功能进行了分析,以期为解析PR10蛋白家族基因调控月季花朵衰老的分子机制提供新的理论依据,为月季花朵品质改良分子育种提供基因储备.
全文HTML
-
月季切花采自中国农业大学月季种质资源圃.按商品月季切花采收标准采收(参考Ma等2005年制定的标准)[12].月季切花采收后立即插入水中,并在1 h内运回实验室,为防止气栓形成,需在水中复剪至25~30 cm,并保留2~3片复叶,平衡2 h备用.拟南芥(Arabidopsis thaniana)为Columbia(Col)生态型.
大肠杆菌(Escherichia coli)DH5α、DNA片段纯化回收试剂盒、质粒提取试剂盒、5'-Full RACE kit、pMD18-T载体、M-MLV反转录酶等购自Takara公司;引物由三博远志公司合成;DNA测序由天一辉远公司完成;其他常规试剂均为国产或进口分析纯.
-
月季花瓣总RNA提取采用Hot Borate法,并做部分修改[17].拟南芥叶片总RNA提取采用TaKara公司TRIzol试剂盒. RACE cDNA克隆方法参考Takara公司BD SMARTTM RACE cDNA Amplification Kit使用方法[18].引物见表 1.
-
基因克隆参考赵福佳等[19]以及李胜等[20]方法,并稍作修改.
RhPR10.2基因3'端序列克隆:通过实验室月季花瓣响应乙烯数据库(htt://bioinfo.bti.cornell.edu/rose)RU05592提供的已有序列设计正向特异上游引物GSP1与GSP2,利用3'-RACE方法及试剂盒提供的两条下游引物AP1与AP2进行巢式PCR克隆获得3'端特异序列;第一轮PCR引物分别为GSP1与AP1,反应体系为20 μL,模板为月季6级花瓣cDNA,反应条件为:95 ℃ 5 min;95 ℃ 30 s,60 ℃ 30 s,72 ℃ 1 min (30循环);72 ℃ 10 min.第二轮PCR引物为GSP2与AP2,反应体系为20 μL,以稀释50的第一轮产物为模板;反应条件为:95 ℃ 5 min;95 ℃ 30 s,60 ℃ 30 s,72 ℃ 1 min (30循环);72 ℃ 10 min.
RhPR10.2基因5'端序列克隆:根据克隆得到的RhPR10.2基因序列设计两条反向特异下游引物GSP3与GSP4,利用5'-RACE kit试剂盒提供的5' RACE outer primer及5' RACE inner primer进行巢式PCR扩增5'端特异序列;第一轮PCR引物为GSP3与5' RACE outer primer,模板为月季6级花瓣cDNA,反应体系为20 μL,反应条件为:95 ℃ 5 min;95 ℃ 30 s,60 ℃ 30 s,72 ℃ 1 min (30循环);72 ℃ 10 min.第二轮PCR引物为GSP4与5' RACE inner primer,以稀释50的第一轮产物为模板,反应体系为20 μL,反应条件为:95 ℃ 5 min;95 ℃ 30 s,60 ℃ 30 s,72 ℃ 1 min (30循环),72 ℃ 10 min.
RhPR10.2基因ORF(开放阅读框)全长校正:利用DNAMAN 5.0软件进行全长拼接并进行ORF(开放阅读框)全长预测,设计两条特异引物GSP5(正向)、GSP6(反向),使用高保真性PrimeSTAR HS DNA聚合酶进行ORF全长校正,PCR反应条件:95 ℃ 5 min;95 ℃ 30 s,60 ℃ 30 s,72 ℃ 1 min (30循环),72 ℃ 10 min.
基因测序:将含有目的片段的回收产物连接到T载体并转化大肠杆菌DH5α,在添加AMP(氨苄)的培养基上37 ℃过夜培养,挑选阳性单克隆送至天一辉远公司测序.
-
qRT-PCR试验采用ABI PRISM Step ONE Plus Real-time PCR仪器;反应体系为20 μL,即2×Mix 10 μL、上下游引物(RhActin5为内参基因)各0.4 μL、模板2 μL、ddH2O 7.2 μL;反应程序为95 ℃ 10 min→95 ℃ 15 s→60 ℃ 1 min(40个循环);数据处理处理采用双ΔΔT方法计算.
-
通过DNAMAN 5.0软件将ORF核苷酸序列翻译成氨基酸序列,利用ProtParam在线工具(http://web.expasy.org/protparam)进行蛋白特性分析;通过Clustal软件进行RhPR10.2同源蛋白序列比对分析;利用MEGA 5.0软件进行系统进化树分析.
-
过表达载体构建:分别设计带有Xba Ⅰ以及Hind Ⅲ酶切位点的正反向引物序列GSP7、GSP8,使用高保真性PrimeSTAR HS DNA聚合酶进行PCR扩增,模板为月季6级花瓣cDNA,PCR反应条件:95 ℃ 5 min;95 ℃ 30 s,60 ℃ 30 s,72 ℃ 1 min (30循环),72 ℃ 10 min;将获得的条带回收并使用Xba Ⅰ以及Hind Ⅲ双酶切,构建到已经双酶切后的Super 1300载体上,获得pSuper1300-RhPR10.2过表达载体.农杆菌转化采用冻融法,拟南芥植株转化采用花序浸蘸法[18].
-
VIGS载体构建:选择RhPR10.2基因保守的318 bp 3'-UTR片段,设计带有Xba Ⅰ和Kpn Ⅰ限制性内切酶的正反向引物,构建pTRV-RhPR10.2沉默载体.病毒诱导的基因沉默(VIGS)试验参考Wu等[16]的方法,并稍加修改.
1.1. 试验材料
1.2. 总RNA提取与反转录
1.3. 基因全长克隆
1.4. qRT-PCR分析
1.5. 蛋白结构及系统进化树分析
1.6. RhPR10.2异源过表达
1.7. VIGS载体构建及RhPR10.2基因沉默
-
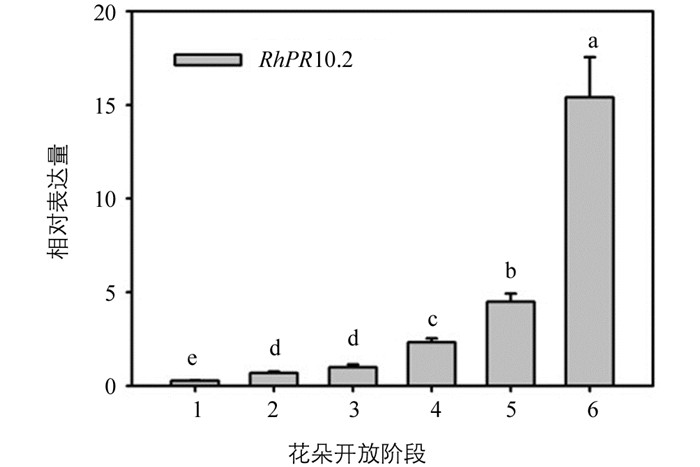
采用qRT-PCR方法检测了RhPR10.2基因在不同开放阶段(1至6级)月季花瓣中的表达谱(图 1).如图所示,月季RhPR10.2基因受花瓣衰老显著诱导.
-
通过RACE PCR方法获得RhPR10.2基因全长ORF序列483 bp,编码160个氨基酸序列(图 2).
-
蛋白特性分析表明月季RhPR10.2蛋白分子式为C796H1250N204O239S2,分子量为17 566.02,半衰期在酵母中大于20 h,大肠杆菌中大于10 h,亲水性系数(GRAVY)为-0.269,为疏水性蛋白;RhPR10.2蛋白肽链正电荷残基(Asp+Glu)为22,负电荷残基(Arg+Lys)为19,等电点(PI)为6.07. RhPR10.2氨基酸序列与月季(Rosa hybrida)、欧洲白桦(Betulapendula)、白羽扇豆(Lupinusluteus)、唐松草(Thalictrum aquilegifolium)、拟南芥(Arabidopsis thaliana)的同源基因序列比对表明,月季RhPR10.2具有PR-10家族蛋白相对保守的P-Loop(GxGGxGxxK)基序,可能与其他物种PR-10蛋白具有相似的蛋白特性(图 3).
-
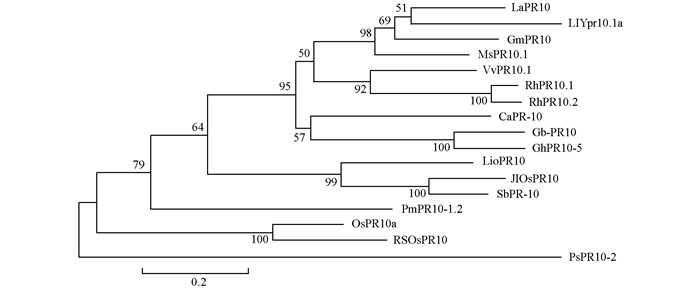
将RhPR10.2蛋白与其他植物,包括白羽扇豆LaPR10、黄羽扇豆LlYpr10.1a、大豆GmPR10、苜蓿MsPR10.1、葡萄VvPR10.1、月季RhPR10.1、甜椒CaPR-10、海岛棉Gb-PR10、陆地棉GhPR10-5、麝香百合LloPR10、水稻JIOsPR10、高粱SbPR-10、白山松PmPR10-1.2、稻谷OsPR10a、罂粟PsPR10等进行系统进化树分析,结果表明RhPR10.2与月季RhPR10.1以及葡萄VvPR10.1亲缘关系最近(图 4).
-
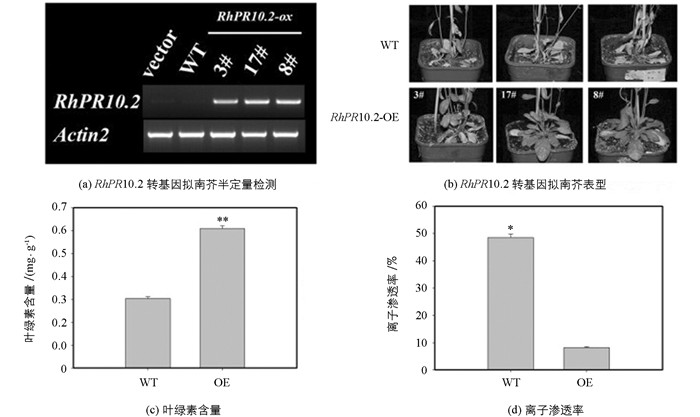
为解析RhPR10.2基因生物学功能,采用花序浸蘸法将pSuper1300-RhPR10.2载体转移进入拟南芥野生型植株,利用潮霉素进行阳性筛选,并进行RT-PCR检测,获得T2代RhPR10.2过表达纯合子株系,分别为3#、17#、8#(图 5a).表型分析发现,过表达RhPR10.2转基因拟南芥表现出显著延缓叶片衰老表型(图 5b).衰老相关指标检测发现,与野生型植株(WT)相比,转基因株系叶绿素含量显著增加,然而离子渗透率显著降低(图 5c,d).
-
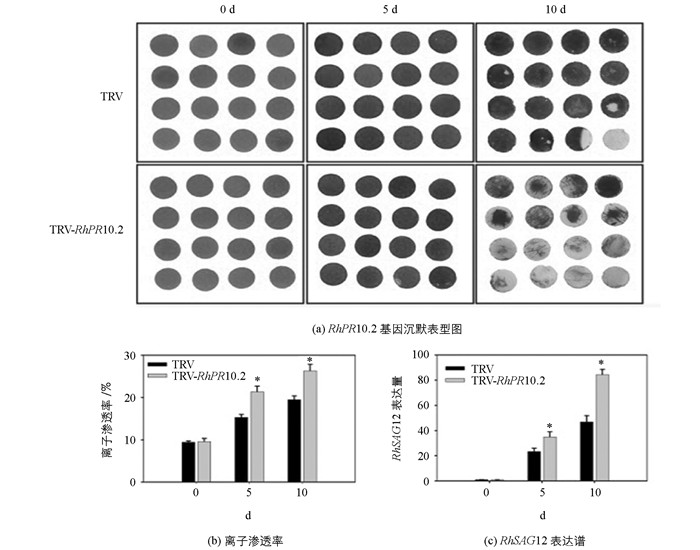
为进一步明确RhPR10.2在月季花瓣衰老调控中的生物学功能,我们构建了VIGS(病毒诱导的基因沉默)载体pTRV-RhPR10.2,采用Wu等[16]的方法在月季花瓣discs中进行了RhPR10.2基因沉默处理.结果表明,较TRV对照相比,沉默RhPR10.2基因显著加速月季花瓣discs衰老进程(图 6 a),同时伴随着更高的离子渗透率(图 6 b)以及衰老marker基因RhSAG12的表达(图 6 c).
2.1. 月季RhPR10.2基因表达谱分析
2.2. 月季RhPR10.2基因ORF全长克隆
2.3. 月季RhPR10.2蛋白特性及同源序列比对分析
2.4. 月季RhPR10.2蛋白系统进化树分析
2.5. RhPR10.2基因异源过表达分析
2.6. RhPR10.2基因沉默分析
-
在前期工作中,我们已经证明了一个受衰老和乙烯诱导的月季RhPR10.1基因,通过影响月季花朵内的细胞分裂素含量进而调控月季花朵衰老[16].为进一步解析RhPR10.1同源基因在月季花瓣衰老中的作用机制,本研究从月季‘萨曼莎’花瓣中克隆得到一个RhPR10.1同源基因RhPR10.2,并对其编码蛋白的特性及生物学功能进行了分析.研究表明,RhPR10.2基因表达量受月季花瓣衰老显著诱导,ORF(开放阅读框)为483 bp,编码160个氨基酸,含有PR-10家族高度保守的P-loop基序,该基序可能与核糖核酸酶活性[22]、亲水配基(细胞分裂素与油菜素内酯)绑定功能[22]以及次生代谢生物酶功能[23]相关.系统进化树分析表明,该蛋白与葡萄VvPR10.1蛋白亲缘关系最近.
从第一个PR-10家族蛋白发现至今[24],人们已对其在不同植物抗病中的功能进行了大量研究[25-26];然而某些植物的PR-10家族蛋白呈组成型表达模式,推测可能参与植物的生长发育调控[21, 30].在油菜(Brassica nupus)中异源过表达一个豌豆(Pisumsativum L.)的PR 10蛋白,转基因植株在盐胁迫条件下的萌发率显著增加[27].在野生型拟南芥中异源过表达ABR17 (一个PR10蛋白)也能增强盐胁迫以及低温胁迫下的萌发率[28].此外,烟草中过表达一个花生(Arachishypogaea)的PR10蛋白基因AhSIPR10后,T1代转基因烟草展现出增强的盐胁迫、重金属胁迫以及干旱胁迫耐性[29].本研究表明,异源过表达月季RhPR10.2基因的拟南芥(T2)纯合子植株,叶片衰老被显著延迟,伴随着更高的叶绿素含量及更低的离子渗透率.与之相反,运用VIGS方法在月季花瓣中沉默RhPR10.2基因,其花瓣的衰老进程被显著提前,且伴随着更高的离子渗透率及衰老marker基因RhSAG12的表达.
本研究对月季RhPR10.2的蛋白特性及生物学功能进行探索,为解析PR-10家族基因在植物衰老中的调控作用提供了参考,但RhPR10.2基因上游调控机制仍需进一步研究.



 下载:
下载: