-
开放科学(资源服务)标志码(OSID):

-
纹枯病(Rhizoctonia solani Kühn)作为一种水稻的常见真菌性病害,严重威胁着粮食安全. 目前的研究发现,水稻对纹枯病的抗性仅表现为中等水平,且易受环境的影响,迄今尚无稳定高抗的抗源. 水稻纹枯病菌在入侵水稻时,主要是通过气孔或者伤口直接攻击水稻的表皮细胞,进而进入水稻植株体内. 其菌丝在穿过气孔后,可形成侵染垫(卷曲菌丝聚集体),侵染垫能产生降解植物细胞壁的酶,为真菌进入植物组织打开通道[1]. 在进入水稻体内后,纹枯病菌能够产生病原体效应物,这是一类RS(Rhizoctonia solani)毒素,能够破坏水稻组织,加速对水稻植株的入侵和传播[2]. 收获后的菌核遗落在田间,越冬后,在第2年继续形成传染源,这也是纹枯病防治难度大的原因. 在水稻中,目前还没有克隆出有关水稻纹枯病抗性相关的基因. 在Sato等[3]的研究中,利用WSS2(抗病品种)与Hinohikari(感病品种)先自交后回交,鉴定到qSB-3和qSB-12两个主效数量性状基因座(Quantitative trait locus,QTL). 另外两个纹枯病抗性qSB11和qSB9,均来自供体Lemont(抗病品种). 向珣朝等[4]的研究中发现了Rsb-2(t),一个来自于3号染色体的纹枯病抗性主效QTL.
细胞色素P450(cytochrome P450)是一个十分古老的基因家族,在动物、植物、真菌和细菌的细胞内广泛存在,在生物体内的细胞色素P450是一类能够与细胞器膜(内质网、线粒体、高尔基体、质体等)结合的且具有混合功能的血红素氧化酶系,是近年来研究的热点之一. 在水稻的防御机制中,CYP450有着不可替代的作用. CYP71Z2在水稻的防御机制中起作用,该基因能够通过IAA途径介导对白叶枯病的抗性[5]. HAN1在调控植物的逆境应答(冷胁迫和低温胁迫)机制中起作用,在JA途径中催化活性茉莉酸-L-异亮氨酸的转化,负调控水稻的耐寒性[6]. CYP94C2b和HAN1一样,也通过催化活性茉莉酸-L-异亮氨酸的转化,进而降低水稻对外源JA的响应和损伤的应答[7]. OsSL编码的CYP71P1蛋白具有色胺5羟化酶活性,在色胺转变成血清素的过程中起催化作用,血清素能够诱导防御基因的表达在水稻防御机制中发挥作用[8]. 此外,外源5-羟色胺诱导水稻防御基因的表达和细胞的程序化死亡,并增加水稻对稻瘟病的抗性. CYP76M7在抗真菌素中起作用,在水稻第2个二萜类生物合成基因簇中起决定作用. 这个基因簇是多功能的,包括合成两类不同植保素(抗真菌素和抗细菌素)的酶,并且通过不同的转录调控相应的基因[9]. CYP81A6具有对苯达松和磺脲类除草剂的抗性[10]. 在CYP72A31的作用下,双草醚和苄嘧磺隆能够被代谢为一种毒性更小的复合物,进而提高拟南芥和水稻对抑制剂类除草剂(乙酰乳酸合成酶)的耐受性[11].
在前期研究中,通过EMS化学诱变处理籼稻保持系西农1B获得了4个遗传稳定的水稻矮化易感纹枯病的突变体,dssb1,dssb1-1,dssb1-2和dssb1-3. 本研究通过构建F2群体进行连锁分析,采用图位克隆的方法精细定位目标基因,并通过形态学分析、组织化学切片、目标基因的功能分析等手段对该突变体进行表型鉴定及目的基因的功能分析,更深入地了解该基因的分子调控机制,以期为水稻抗病育种提供参考.
全文HTML
-
水稻矮化纹枯病易感突变体dssb1,dssb1-1,dssb1-2和dssb1-3,由西南大学水稻研究所培育的保持系西农1B(籼稻)经过甲基磺酸乙酯(EMS)诱变剂处理得到的. 白叶枯病菌株(zhe-173),中国水稻研究所侯雨萱老师馈赠;纹枯病菌株(RH-9),扬州大学潘学彪老师提供;大肠杆菌(Escherichia coli)DH5α感受态细胞原液,购自北京TransGen Biotech技术有限公司;PAN580亚细胞定位质粒,南京农业大学万建民实验室馈赠.
-
水稻种植于西南大学歇马镇水稻基地,按常规模式进行田间管理. 成熟后,随机收获10株,对主要的农艺性状进行考察,调查结果进行t检验.
-
参照潘学彪等[12]的短牙签嵌入法接种水稻纹枯病菌,接种后的第7 d,14 d对野生型和突变体的发病情况进行统计.
水稻白叶枯病抗性的鉴定方法:将在斜面培养基上已活化的病菌刮取一环接种至培养基中,置于28 ℃,120 r/min的摇床培养24~48 h,采用麦氏比浊法配制3×108 CFU/mL菌液浓度,用灭菌剪刀蘸取上述菌浮液,斜剪去叶尖1.5 cm,每株处理5个叶片,接种后的第7 d和14 d,对野生型和突变体白叶枯病的发生情况进行调查.
-
冷冻切片:抽穗期(叶片形态稳定),分别剪取野生型(WT)和突变体倒1叶叶中部约1 cm长的叶片(对应的同一部位),放入提前装有包埋剂(Tissue-Tek,SAKURA)的离心管中包埋,待包埋剂完全凝固时取出,然后用冷冻切片机切成25 μm厚,放置于载玻片上用ddH2O反复冲洗直至没有包埋剂残留,轻轻盖上盖玻片,于显微镜下观察并拍照.
石蜡切片:在灌浆期选取长势一致的野生型和突变体材料固定于FAA固定液中,依次用不同浓度梯度(50%,70%,85%,95%,100%)的酒精进行脱水处理,重复3次,将经过酒精梯度脱水之后的水稻材料依次用不同体积比(3∶1,1∶1,1∶3)的无水乙醇-二甲苯混合液进行透明处理,将二甲苯与融化石蜡按1∶1进行配比浸入材料中,间隔12 h后换成新的纯石蜡,用镊子将材料轻夹至液体石蜡中,待石蜡凝固,于冰箱-4 ℃放置48 h,将包埋好的蜡块切片8 μm,将切好的蜡带铺于载玻片上,展片后烘片48 h,脱蜡,封片观察.
扫描电镜观察:在水稻抽穗期时,分别随机选取野生型和突变体倒1叶叶片中部,剪取后将其置于有导电胶的底座上,将固定好的样品放置于扫描电子显微镜冷冻台上(快速操作,避免失水皱缩),调整到合适的高度,于-20 ℃迅速冷冻条件下观察水稻叶片的表面.
-
木质素、纤维素、半纤维素质量分数测定方法参见索莱宝木质素、纤维素、半纤维素提取试剂盒.
-
选育遗传稳定的突变体,与“缙恢10号”分别杂交后获得F1种子;将F1种子播种于海南基地并观察F1与亲本的植株表型以确定其显隐性,待其自交后收获F2种子;种植F2群体并对性状分离比进行统计调查,选取与突变体表型一致的植株进行基因定位.
-
参照普罗麦格的试剂盒提取RNA,反转录实验参照TAKARA公司提供的反转录试剂盒上步骤进行,所得cDNA溶液稀释10倍后备用. 以2-ΔΔCt计算各基因的相对表达量,内参基因选用ACTIN进行相关基因cDNA扩增,对每个样品做3次重复. qRT-PCR主要用于定位区间内部分基因和一些防卫反应相关标记基因的表达分析.
-
30 ℃恒温浸泡适量野生型西农1B水稻种子,露白后,将其置于营养土中土培10 d,剥去水稻幼苗的叶鞘,留取幼茎,冲洗干净后将其用刀片切出小段,将小段转移至避光的灭菌三角瓶中. 加入适量的酶解液,抽真空,40 r/min,28 ℃振荡培养4~6 h. 200目细胞过滤筛过滤,留残渣,将剩下的组织转移至新配的W5(154 mmol氯化钠,125 mmol氯化钙,5 mmol氯化钾,2 mmol MES,5 mmol葡萄糖,pH值为5.7)中,于80 r/min的摇床上培养1 h. 200目细胞过滤筛过滤,留滤液,加入5 mL的W5,200 g离心5 min,在上清中可看到明显的一层原生质体,吸取并转移至新的离心管中. W5漂洗,180 g离心5~10 min,去除上清. 加入MMG(0.6 mol甘露醇,15 mmol氯化镁,4 mmol MES,pH值为5.7)溶液进行重悬,移液枪轻轻吹打. 向离心管中依次加入10 μg质粒,制备好的原生质体,PEG溶液(0.2 mol甘露醇,100 mmol氯化钙,40%PEG4000,pH值为5.7),温和混匀,室温避光条件下进行20 min的静置培养. 加入440 μL W5溶液终止反应,轻柔混匀,1 500 r/min离心3 min,小心弃上清. 用W5溶液将原生质体进行重悬(移液枪轻揉吹打),转移到清洗过的细胞培养板内,室温下避光培养12 h以上. 激光共聚焦显微镜观察,并拍照保存.
1.1. 实验材料
1.2. 实验方法
1.2.1. 农艺性状考察
1.2.2. 抗病性分析
1.2.3. 组织化学切片及扫描电镜观察
1.2.4. 细胞壁成分分析及质量分数测定
1.2.5. 遗传学分析
1.2.6. 相关基因的表达分析
1.2.7. 亚细胞定位
-
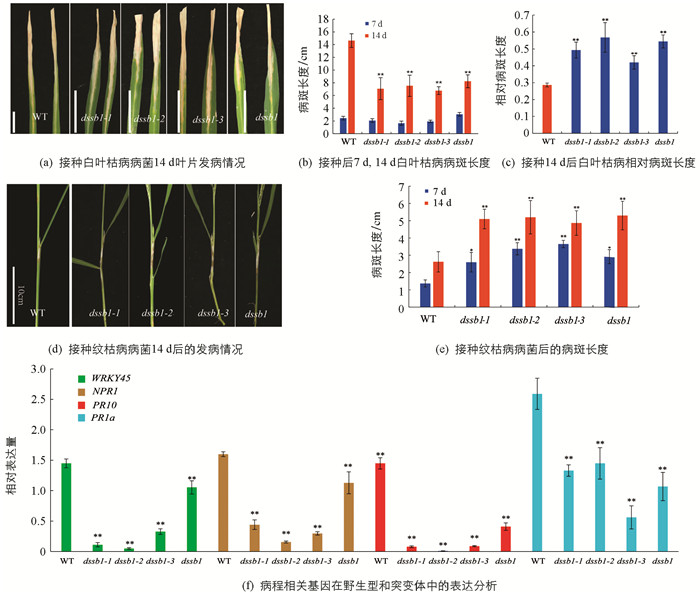
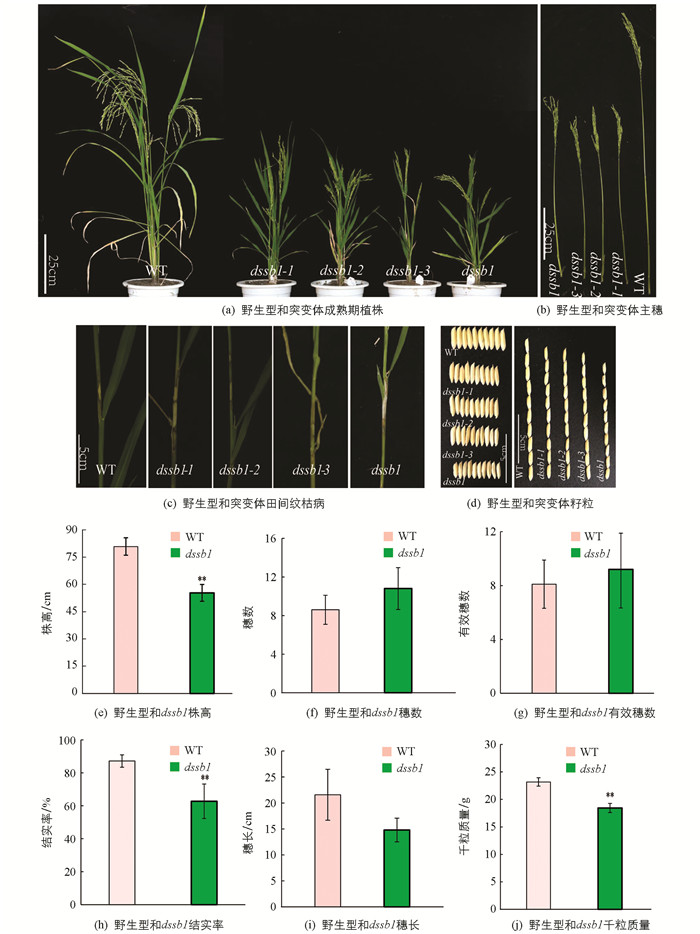
在籼型水稻保持系西农1B的EMS突变库中筛选到4个稳定遗传的矮化且纹枯病易感的突变体,命名为dssb1,dssb1-1,dssb1-2,dssb1-3. 在田间种植的条件下发现,相较于野生型,4个突变体在整个生育期都表现为植株矮化(图 1a,b),植株的节间变短,且籽粒都不同程度的变小(图 1d),其中突变体dssb1的籽粒最小,其粒长和粒宽均极显著低于野生型,而dssb1-1,dssb1-2和dssb1-3这3个突变体之间籽粒相差不大;同时这4个突变体对纹枯病的抗性降低,在野生型的叶鞘处仅出现一小块纹枯病病斑,而突变体的叶鞘出现大块病斑,在出现病斑处已出现不同程度的干枯(图 1c).
在成熟期,收取野生型(WT)和突变体dssb1各10株并对其农艺性状进行统计(因这4个突变体表型基本一致,所以仅仅只对dssb1和野生型的农艺性状进行考察). 结果发现,dssb1的株高、穗长、结实率、千粒质量均降低,其中株高、结实率、千粒质量分别降低了31.5%,28.1%,20.4%(图 1e,h,j),差异有统计学意义,而穗长差异无统计学意义(图 1i). 穗数和有效穗数较野生型有所增加,但差异无统计学意义(图 1f,g).
-
在分蘖期,接种白叶枯病菌后第7 d调查统计发现,野生型与突变体差异无统计学意义,但在第14 d,发现野生型的病斑长度较突变体的病斑长度长(图 2a,b). 因突变体的叶子变小,对其相对病斑长度进行统计发现,野生型的相对病斑长度明显短于突变体,其中dssb1-2发病最严重(图 2c),说明突变后,突变体对白叶枯病的抗性降低. 在孕穗期,接种纹枯病菌后的第7 d,14 d,对其发病情况进行调查统计. 结果显示,在接种部位均出现了纹枯病病斑,相较于野生型,突变体病斑的长度比野生型的病斑长度长(图 2d). 第7 d后,突变体的病斑扩大速度明显快于野生型(图 2e). 突变体植株明显矮于野生型,进一步说明突变体对纹枯病的抗性降低. 在这两个不同背景下的不同位置的突变均是如此,说明该基因的突变导致对纹枯病的抗性降低.
此外,未接种纹枯病菌的条件下,这4个突变体也表现出对纹枯病的抗性降低. 我们对野生型和突变体中病程相关基因进行了表达分析. 结果显示,相比于野生型,病程相关基因NPR1,PR1a,PR10以及WRKY45的表达量在等位突变体中显著降低,且差异具有统计学意义(图 2f).
-
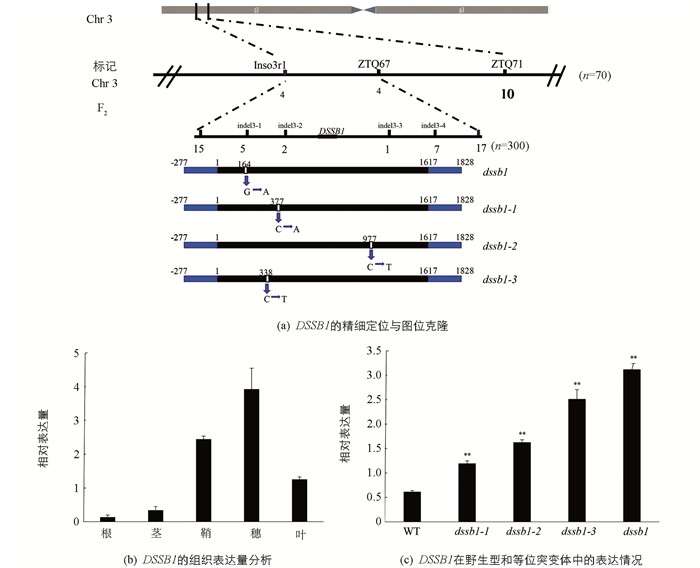
为了确定其遗传方式,我们分别以dssb1,dssb1-1,dssb1-2和dssb1-3为母本,恢复系缙恢10号(J10)为父本进行杂交,其F1均表现正常. 同时分别将这4个F1种子自交收获F2群体,对F2群体的性状分离进行调查统计,这4个F2群体的性状比符合3∶1的孟德尔遗传分离比,表明这4个突变体受1对隐性核基因控制. 利用均匀分布在水稻12条染色上的96条西农1B与缙恢10号差异引物,对这4个F2群体构建的正常DNA基因池、突变体DNA基因池进行多态性分析,发现在第3条染色体上的ZTQ67,ZTQ71,Inso3r1引物疑似连锁. 进一步利用70株F2群体对连锁点进行验证,确认在该位置显示连锁. 利用实验室现有的西农1A与缙恢10号的差异序列进行indel引物设计,并扩大群体,最终将目的基因DSSB1锁定在indel3-2与indel3-3引物之间,物理距离约为78Kb(图 3a). 对区间内的所有基因进行PCR扩增测序,结果发现,这4个突变体均在LOC_Os03g04680编码框中的不同位置发生了碱基的突变. dssb1在距离起始密码子164 bp处发生了1个碱基的替换,由G突变为A,导致其编码的氨基酸由脯氨酸(pro)变为亮氨酸(leu). dssb1-1的LOC_Os03g04680编码框在377 bp处发生了1个碱基的替换,由C突变为A,其编码的氨基酸由甘氨酸(Gly)突变为丙氨酸(Ala). dssb1-2的LOC_Os03g04680编码框在977 bp处发生了1个碱基的替换,由C突变为T,其编码的氨基酸由色氨酸(Trp)变为了终止密码子,导致翻译的终止. dssb1-3的LOC_Os03g04680编码框在338 bp处发生了1个碱基的替换,由C突变为T,导致其编码的氨基酸由甘氨酸(Gly)变为了天冬氨酸(Asp)(图 3a).
在抽穗期,对该候选基因DSSB1在野生型的根、茎、鞘、穗、叶等部位进行qRT-PCR分析. 结果显示,DSSB1在野生型的各个部位均有表达,但在穗中的表达量明显高于其他部位,其次是鞘与叶,在根和茎中的表达量较低(图 3b). 对突变体植株dssb1,dssb1-1,dssb1-2,dssb1-3和野生型(WT)进行候选基因DSSB1的qRT-PCR分析,结果显示,相比于野生型,在这4个突变体内,该候选基因的表达量明显上调,且差异有统计学意义(图 3c).
-
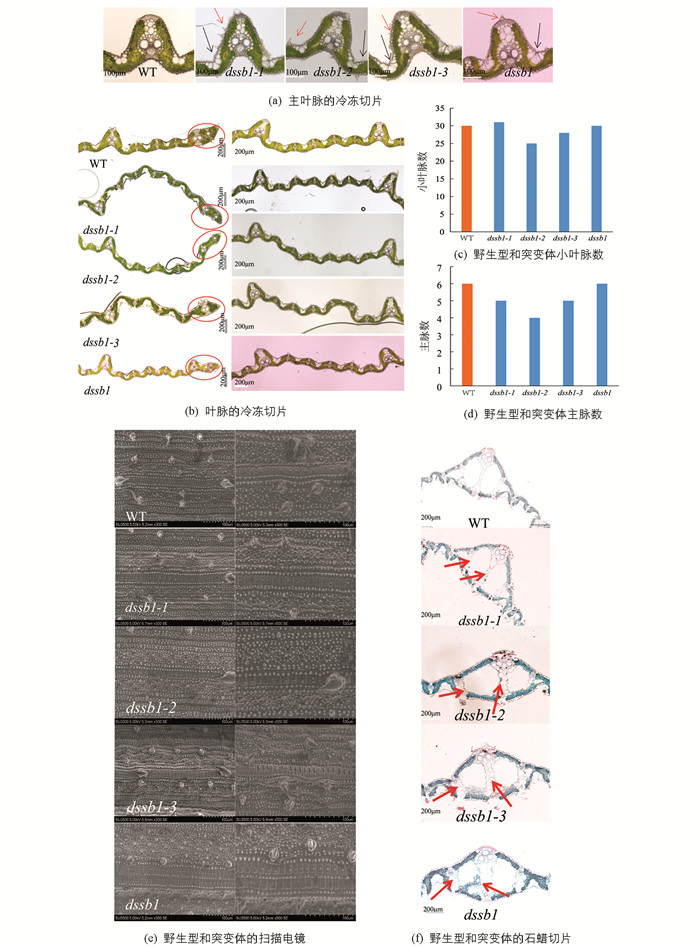
在抽穗期,待叶片发育稳定后,对dssb1及其等位突变体的倒1叶中部叶片进行冷冻切片,对叶片的主脉及叶中脉进行统计分析后发现,这4个突变体的表面硅化细胞、泡状细胞及叶边缘均发育异常(图 4a,b). 小叶脉的数量除dssb1-1外,均较野生型少(图 4c);主脉数量除dssb1外,其余突变体均少于野生型(图 4d).
在抽穗期,对野生型西农1B及其突变体dssb1-1,dssb1-2,dssb1-3和dssb1的倒1叶中部叶片进行扫描电镜观察发现,与野生型相比,各等位突变体叶片表面的硅化细胞的数量明显增多,且分布密集、杂乱,这与冷冻切片结果一致. dssb1-3与dssb1叶片表皮的硅化细胞数量最多,分布最为密集,分析可能是其在不同位点发生突变,导致其蛋白行使功能的能力不一(图 4e).
对叶片进行石蜡切片发现,dssb1-1,dssb1-2,dssb1-3和dssb1突变体的维管束发育均与野生型不一致,均发育异常,同时石蜡切片结果显示,叶片的通气组织也发育异常(图 4f).
-
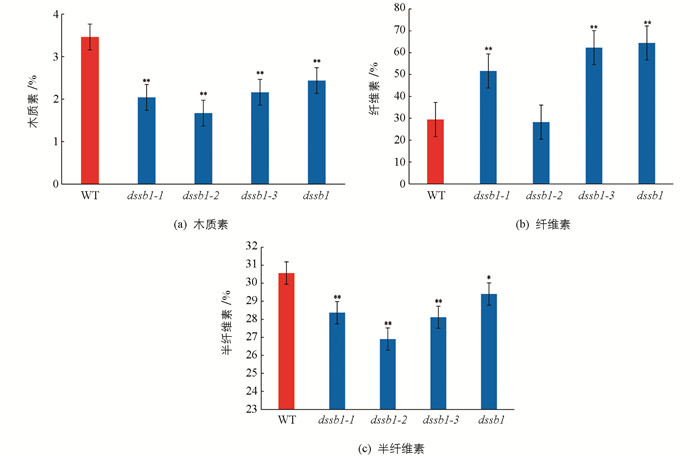
对dssb1及等位突变体扫描电镜分析发现,在不同的等位突变体叶片表面均出现硅化细胞的数量增多,所以我们对野生型与等位突变体叶片的细胞壁成分进行了测定. 结果发现,相比于野生型(WT),dssb1及其等位突变体的木质素质量分数显著低于野生型,且差异有统计学意义(图 5a);除dssb1-2外,其余等位突变体的纤维素质量分数均高于野生型,且差异有统计学意义(图 5b);半纤维素和木质素质量分数的变化趋势基本一致(图 5c).
-
为了确定DSSB1蛋白的亚细胞定位,我们构建了DSSB1-GFP融合表达载体,通过PEG介导转化的方法导入水稻原生质体中,使其在水稻原生质体中瞬时表达,观察到DSSB1-GFP绿色荧光信号可以跟内质网marker红色荧光信号完全重叠(图 6),结果显示,DSSB1是一个内质网蛋白.
2.1. dssb1等位突变体的表型鉴定及农艺性状考察
2.2. dssb1及等位突变体的抗病性分析
2.3. dssb1及其突变体的遗传分析及基因定位
2.4. dssb1及其等位突变体的组织化学切片及扫描电镜观察
2.5. dssb1及等位突变体木质素、纤维素、半纤维素的质量分数分析
2.6. DSSB1亚细胞定位
-
在本研究中,我们在水稻EMS诱变的突变体库中,鉴定到了4个矮化纹枯病易感突变体. 遗传分析表明,这4个突变体均受1对隐性核基因控制,精细定位显示这4个突变体是在LOC_Os03g04680的编码框的不同位置发生突变,与之前所报道的突变位点均不一致. 这4个突变体全生育期均表现为植株矮化、籽粒变小. 在Zhang等[13]的研究中也有相关表型的报道,其突变体sd37表现出植株矮化、叶片和籽粒变小,其研究是通过减少细胞的数量,进而调控水稻的株高,而本研究中是通过调控细胞的长度进而影响植株的矮化. 在Zhang等[13]的研究中,还报道了该基因可能在调控蜡质上的合成中起作用,bsh1叶鞘中角质层蜡质层的质量分数相比于野生型显著降低. 在Wang等[14]的研究中,也报道了该基因可能在蜡质合成中以未知的调控机制发挥作用,同时其研究还发现bsh1表达异常可引起细胞壁组分异常. 本研究中,这4个突变体在细胞壁的成分上均出现不同程度的变化,分析是其突变位点的不一致导致其合成蛋白的空间结构发生了改变,进而影响该蛋白功能的正常行使. 研究表明,CYP450基因主要通过转录水平进行调节,以mRNA的剪切和蛋白翻译等转录后调节为辅. 在Ramamoorthy等[15]的研究中,OsCYP96B则是通过转录剂量对水稻的株高进行调节.
OsCYP96B4编码的是一个细胞色素P450单加氧酶,属于CYP96家族. 细胞色素P450是生物体内一大类酶,其作用范围广泛,在植物的生长发育中起着重要的调节作用. 本研究除发现前人所报道的表型外,还发现了其在植物的防御机制中起作用,在田间自然条件下,这4个等位突变体表现出纹枯病发病严重. 在接种水稻纹枯病菌和白叶枯病菌的条件下,相对于野生型,这4个突变体对白叶枯病和纹枯病的抗性降低,感病严重. 矮化育种与抗病育种作为育种家们一直研究的热点,矮秆资源和抗病资源的结合应用是提高水稻优质高产的必要条件. 本研究中的dssb1及其等位突变体在整个生育期均矮化;在田间种植时,纹枯病自然发病严重;接种水稻纹枯病菌和白叶枯病菌后,调查统计发现纹枯病和白叶枯病均发病严重,表明dssb1-1,dssb1-2,dssb1-3和dssb1突变体可能是OsCYP96B4的新等位突变体.
株型和抗病是水稻优质高产的两个决定性关键因素,挖掘水稻矮化抗病资源具有十分广阔的应用前景. 随着相关技术上的突破,越来越多的既控制株型同时又在植物防御机制中发挥作用的基因被报道. 在Sato等[16]的研究中,报道了一个抗病的QTL位点qSB-3,该位点位于水稻3号染色体SSR标记的RM3856附近,且qSB-3与控制株高的qCL-3的位置重叠,表明qSB-3的抗病效应可能受株高的影响. 在Zhang等[17]的研究中,揭示了OsALDH2B1和OsLIC可能存在两条途径在水稻的防卫机制中发挥作用,而OsLICs是一个分蘖角度增加调控子,其表达受BR的调控,抑制OsLIC的表达,会导致植株的矮化、叶片夹角和分蘖角增大,且产量降低. 在Li等[18]的研究中,报道了GH3-8在调控水稻的株型和防御机制上发挥着重要作用,GH3-8编码一个吲哚乙酸氨基化合成酶,在维持体内游离IAA的动态平衡中发挥作用,抗病水稻品种中,在病原菌入侵部位会诱导GH3-8的快速表达进而增强植株对病菌的耐受性. GID1不仅可以通过GA途径对水稻株高进行调控,同时也会抑制水稻体内SLR1的活性. 在突变体gid1中,PBZ1和PR10蛋白的质量分数增加[19]. OsLOL2的RNAi植株表现出对白叶枯病的耐受性降低,内源活性GA1质量分数降低,但在外源GA3后矮化表型恢复,而OsKS1的表达量下降[20]. IPA1正调控水稻株型基因DEP1,在对水稻株高进行调控的同时增强免疫力[21]. 矮化育种作为主要的育种方式,一直都是育种家们研究的热点,但在密植的同时,也就意味着更容易感病. 在前人的研究中,已经有过水稻株高与抗性相关的报道. 株高在水稻“避病”机制中扮演着关键性的角色,植物在形态建成的过程中,通过改变自身的形态来获取对自身有利的生长条件——避病,趋益[22]. 还有一些研究则报道了抗性相关QTL与株高相关QTL的共定位[23]. 某些抗病基因的实质就是通过改变其株型的特征,进而改变植株本身和群体形成的空间环境,且此空间的微环境不利于病原菌的生长繁殖而利于植株的生长,从而减少了病原菌的侵害. DSSB1所编码的是一个细胞色素P450单加氧酶OsCYP96B4,该基因具有“一因多效”性,在调控水稻的株高、次生细胞壁的形成以及参与脂类代谢并调控细胞的伸长等生物进程中起作用,但对于该基因可能在调控水稻的抗病机制方面并未有相应的报道. 本研究中所报道的4个等位突变体除了矮化外,均表现为对纹枯病、白叶枯病的抗性降低. 白叶枯病菌在入侵水稻后,通过T3SS分泌系统将效应蛋白注入到寄主植物细胞内,这是一种侵染水稻维管束的革兰氏阴性细菌,其能在水稻叶尖和叶的边缘产生黄绿色的斑点,随后侵染至木质部的导管并导致叶片枯萎变为灰白色. 本研究中,在接种水稻白叶枯病菌14 d,发现突变体白叶枯病发病严重;对突变体的叶片进行石蜡切片发现,其维管束和通气组织发育异常;冷冻切片显示,叶片边缘发育异常. 这可能是白叶枯病菌接种后,突变体对白叶枯病抗性降低所至. 在Talbot等[24]的研究发现,立枯丝核菌菌丝分泌的细胞壁降解酶(果胶酶、漆酶和木聚糖酶)可以将水稻细胞壁的复杂大分子如纤维素、半纤维素和果胶等分解成单糖,从而促进菌丝的侵入. 本研究中dssb1及其等位突变体由于其突变位点的不一致,导致细胞壁组分发生了改变,木质素和半纤维素质量分数均降低,而纤维素的质量分数升高. 水稻纹枯病菌主要通过产生细胞壁降解酶,软化和降解植物细胞壁,致病力越强的菌株分泌的酶活力越高. 本研究中所用的纹枯病病菌RH-9属于强致病力病菌,在接种后更易入侵进入植株. qRT-PCR结果也显示,在突变体中,病程相关基因NPR1,PR1a,PR10,WRKY45的表达量显著降低,进一步说明DSSB1在植株的抗病机制中可能起着调控作用. 在Wang等[25]的研究中,OsCYP96B4的表达异常会影响细胞壁相关基因的表达,会导致细胞壁组分的改变,这也为解释OsCYP96B4在抗病机制中起着重要的调控机制提供了可能性.



 下载:
下载: