-
桑树(Morus alba L.)适应性强,几千年来作为家蚕唯一的饲料源,在全国各省区广泛种植.由于桑树具有耐寒耐旱耐山火,可保持水土[1-2],萌生能力极强,对氯气、二氧化硫和硫化氢具较强耐受性[3]等特性,因而是生态改良的主要树种.桑果不仅基础营养物质含量丰富,而且富含氨基酸、维生素以及白藜芦醇、花青素等抗氧化活性物质[4],具有增强人体免疫力、延缓衰老和美容养颜等功效[5].然而,近年来桑疫病和菌核病等各类病害日趋严重,而现有针对桑树病害的化学农药防治方法所导致的负面影响亦日益凸显,严重制约生态桑产业的健康发展,故寻找高效且绿色环保的桑树病害防治方法已迫在眉睫.
植物内生菌(Endophytes)是生活于健康植物各组织器官的细胞间隙或者细胞内部,并与宿主植物和谐共处的一类微生物[6-7].作为植物微生态系统的重要组成部分,内生菌构筑了宿主植物的健康屏障[8-10],能够通过合成抗菌活性物质[11]、竞争性排阻[12]、促进植物生长[13]及诱导抗性[14]等方式增强宿主植物的抗病能力.近几年,对桑树内生菌多样性及利用其进行植物病害生物防治方面已有研究,但主要集中于培养法.路国兵等人[15]从不同桑树品种的不同组织中共分离得到内生细菌229个分离株,并从中筛选得到67个分离株对桑树炭疽病菌或桑粘格孢菌具有拮抗作用;谭广秀[16]从桑枝中分离获得内生细菌26个分离株,并从中筛选出2株具有抑菌活性的内生菌.此外,本研究小组的研究结果表明桑树内生拮抗菌成团泛菌(Pantoea agglomerans)及特基拉芽孢杆菌(Bacillus tequilensis)分别对桑细菌性疫病病原菌、桑椹缩小性菌核病病原菌有稳定而显著的抑制作用[17-18].然而培养法存在一定局限,无法全面客观反映植物内生菌的种群结构.因此,本文采用构建桑树内生细菌16S rDNA文库的非培养法和培养法,对桑树内生细菌的多样性进行系统研究,并检测培养法获得的内生细菌对桑疫病病原菌(丁香假单胞菌桑致病变种)等10株病原指示菌的拮抗作用,以期阐明内生菌与桑树共生过程中的功能和潜在作用,为利用内生菌进行桑树病害的生物防治提供新的资源.
全文HTML
-
健康桑树桐乡青于2013年7月取自西南大学生物技术学院实验桑园“桑之源”.
-
丁香假单胞菌桑致病变种(Pseudomonas syringae pv. mori)、核盘菌(Sclerotinia sclerotiorum)、终极腐霉(Pythium ultimum)、炭疽病菌(Colletrichum lagenarium)、腐皮镰刀菌(Fusarium solani)、疫霉菌(Phytophthora nicotianae)、立枯丝核菌(Rhizoctonia solani)、链格孢菌(Alternaria sp.)、轮枝菌(Verticillium dahliae)、纹枯病菌(Thanatephorus cucumeris),均为本研究小组收集保存.
-
马铃薯葡萄糖琼脂培养基(PDA):马铃薯200.0 g,葡萄糖20.0 g,琼脂15.0 g,蒸馏水1 000.0 mL,pH自然;马铃薯葡萄糖液体培养基(PDB):马铃薯200.0 g,葡萄糖20.0 g,蒸馏水1000.0 mL,pH自然;高氏Ⅰ号培养基(GA):可溶性淀粉20.0 g,KNO3 1.0 g,NaCl 0.5 g,K2HPO4 0.5 g,MgSO4 0.5 g,FeSO4 0.01 g,琼脂15.0 g,蒸馏水1000.0 mL,pH=7.2~7.4;水琼脂培养基(WA):琼脂15.0 g,蒸馏水1 000.0 mL,pH自然.以上培养基均在121 ℃温度下灭菌20 min备用.
-
基因组提取试剂盒PrepMan Ultra Sample Preparation Reagent购自美国ABI公司;DNA marker、Taq酶、dNTP、10×PCR Buffer、Mg2+、PMD19-T Vector载体购自宝生物工程(大连)有限公司;凝胶回收试剂盒购自AxyGEN公司;PCR引物购自南京金斯瑞生物科技有限公司;其余均为国产分析纯.电泳仪、水浴锅均购自北京六一仪器厂,PCR仪购自美国ABI公司.
-
从实验桑园采集桐乡青的茎,用自来水冲洗干净、晾干、编号后于4 ℃保存,并及时分离内生菌.
桑树茎表面消毒[11]:将桑树枝条剪成长度约5.0 cm的茎段,置于75.0%的乙醇中完全浸湿,取出后于酒精灯上引燃茎表面乙醇,待乙醇燃尽,以此表面消毒的桑枝在空白培养基上滚动,使桑枝每个表面均接触培养基,于22 ℃温度下培养作为空白对照,验证表面消毒是否彻底.
-
采用消毒后桑树茎皮为实验材料,按照文献[19-20]的方法获得桑树皮总基因组DNA,以此作为模板,使用细菌16S rDNA扩增通用引物799F:5′-AACAGGATTAGATACCCTG-3′和1492R:5′-GGTTACCTTGTTACGACTT-3′进行PCR扩增[19-21]. PCR反应体系(25 μL):Premix Taq 12.5 μL,引物各1 μL,DNA 1 μL (20 ng),ddH2O 9.5 μL. PCR反应条件为:95 ℃预变性5 min;94 ℃变性30 s,50 ℃退火45 s,72 ℃延伸1 min,30个循环;72 ℃延伸10 min.
16S rDNA基因文库的构建[20]:PCR产物1%琼脂糖凝胶电泳,将730 bp左右的条带切下,进行回收纯化,产物与PMD19-T Vector载体连接,转化至E.coli DH 5α感受态细胞.在含氨苄青霉素(50 μg/mL)的LB平板上筛选阳性转化子.用菌落PCR的方法,以PMD19-T载体上的M13-47 5′-CGCCAGGGTTTTCCCAGTCACGAC-3′和M13-48 5′-AGCGGATAACAATTTCACACAGGA-3′为引物,验证阳性克隆. PCR反应体系(25 μL):Premix Taq 12.5 μL,引物各1 μL,菌液1.5 μL,ddH2O 9 μL. PCR反应条件为:95 ℃预变性10 min;94 ℃变性1 min,53 ℃退火1 min,72 ℃延伸2 min,30个循环;72 ℃延伸10 min.
桑树皮内生细菌群落16S rDNA文库扩增性rDNA限制性酶切片段分析(ARDRA):取阳性克隆的菌落PCR产物4 μL分别用Hae Ⅲ和Hha Ⅰ内切酶进行酶切分型[19].酶切反应体系(5 μL):Hae Ⅲ或Hha Ⅰ(10 U/μL)0.2 μL,PCR产物4 μL,10× Buffer 0.5 μL,ddH2O 0.3 μL. 37 ℃恒温放置4 h,酶切产物用2.5%的琼脂糖凝胶进行电泳检测.选取两种限制酶的酶切带型不同的克隆送至金斯瑞生物科技有限公司进行测序.
-
将经表面消毒的桑树茎置于无菌培养皿内,用无菌手术刀片分3层剖开,并将所得碎片分别置于PDA培养基、GA培养基和WA培养基表面,22 ℃培养4周,逐日观察.待茎块边缘长出菌落后,采用平板划线法进行纯化培养.
利用基因组提取试剂盒获得菌株DNA,以此为模板,用细菌通用引物27F:5′-AGAGTTTGATCCTGGCTCAG-3′和1492R:5′-GGTTACCTTGTTACGACTT-3′扩增内生细菌16S rDNA序列. PCR反应体系(25 μL):10×PCR Buffer 2.5 μL,Mg2+(1.5 mmol/L)1.5 μL,dNTP(10 mmol/L) 2.0 μL,引物各1.0 μL,DNA 1.0 μL,Taq酶0.1 μL,ddH2O 15.9 μL. PCR反应条件为:95 ℃预变性4 min;94 ℃变性30 s,50 ℃退火45 s,72 ℃延伸1 min,30个循环;72 ℃延伸10 min.扩增产物送至生工生物工程(上海)股份有限公司测序.
-
通过NCBI的VECTER SCREEN去除非培养法测序所得的16S rDNA基因的载体片段[21],将所得到的16S rDNA序列在线进行嵌合序列鉴定(http://comp-bio.anu.edu.au/bellerophon/bellerophon.pl)去除嵌合序列[22].将获得的基因序列用Blast程序(http://blast.ncbi.nlm.nih.gov/Blast.cgi)与数据库中已登记的基因序列进行同源性比较分析,获得菌种的相似度信息
采用分离频率(Relative frequency)、香农多样性指数(Shannon-Wiener Biodiversity index,H′)、辛普森多样性指数(Simpson's Biodiversity index,D)、丰度(Richness)及皮耶诺均匀度指数(Pielou's evenness index,J)5个指标[23]及稀疏曲线(Rarefaction Curve)[24]对两种方法得到的桑树内生菌多样性进行评价分析比较.
-
将分离获得的内生细菌接种于PDB培养基,28 ℃、180 r/min振荡培养96 h;收获发酵液后12000 r/min离心30 min,取上清液备用[17].以桑疫病病原菌丁香假单胞菌桑致病变种(Pseudomonas syringae pv. mori)及其他9种常见植物病原真菌为靶标,灭菌PDB培养液作对照,用抑菌圈法检测桑树内生菌发酵上清液对靶标病原菌的拮抗活性,筛选桑树内生拮抗菌株.
将所得拮抗菌16S rDNA基因序列在GenBank里进行比对(http://www.ncbi.nlm.nih.gov/BLAST),下载GenBank中同源性较高序列,利用CLUSTALX软件进行聚类分析,再利用MEGA4.0软件,使用N-J法进行1 000次步长计算,构建系统发育树[17].
1.1. 材料
1.1.1. 供试桑树材料
1.1.2. 拮抗菌筛选靶标菌株
1.1.3. 培养基
1.1.4. 主要试剂和仪器
1.2. 方法
1.2.1. 样品的采集及处理
1.2.2. 非培养法对桑树内生细菌多样性分析
1.2.3. 培养法对桑树内生细菌多样性分析
1.2.4. 数据分析
1.2.5. 桑树拮抗性内生细菌的发育分析
-
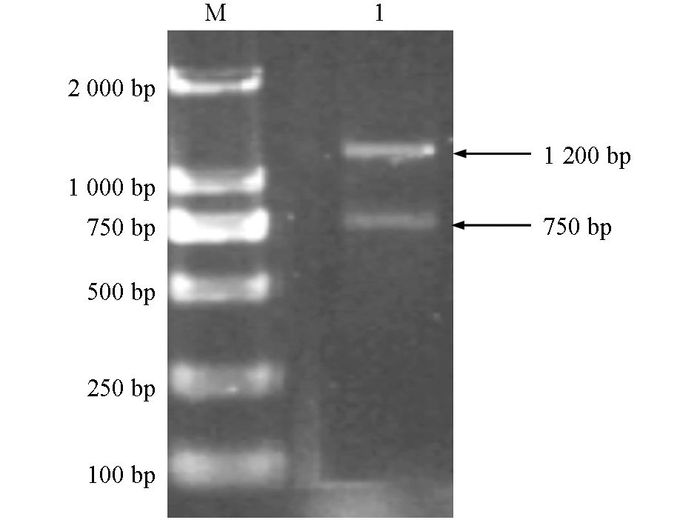
样品总DNA提取和16S rDNA基因的PCR扩增:提取的桑树皮总DNA片段较为完整,基因组片段大于15 kb.通过细菌16S rDNA基因特异性PCR扩增,得到大小为750 bp左右的目的片段,同时还扩增出1 200 bp左右的片段,推测可能是桑树线粒体18S rDNA的部分序列(图 1).经表面消毒的桑枝滚动的培养基在培养后,于22 ℃温度下培养72 h,无菌落生长,说明实验中得到的结果均为桑树内生菌.
16S rDNA基因克隆文库阳性转化子的筛选与酶切分析:从所构建16S rDNA基因文库中随机挑取120个单菌落,用质粒上的通用引物M13-47和M13-48进行菌落PCR扩增,验证阳性克隆,最终共筛选获得111个阳性克隆,阳性克隆大小约为900 bp.
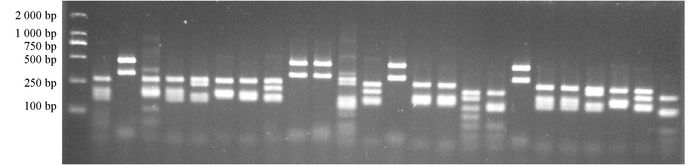
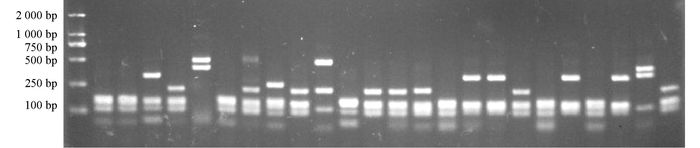
阳性克隆PCR扩增产物首先用内切酶Hae Ⅲ进行酶切,图谱结果显示共有6种带型(图 2),选择带型相同的克隆,将其菌落PCR产物再次使用内切酶Hha Ⅰ进行酶切,验证其是否的确为同一克隆(图 3).由图 2和图 3可见酶切产物得到片段大小在100~700 bp之间,其总和都接近于未消化的PCR产物全长.
经两次酶切比较,共得到40个酶切图谱不同的克隆,将不同的克隆进行测序.根据酶切图谱和克隆测序结果,排除嵌合体后,111个克隆分为22个分类单元(OTUs).测序后将16S rDNA序列提交至NCBI数据库GeneBank获得登录号KT766085-KT766118.经Blast序列比对,111个阳性克隆序列分属于细菌域的3个类群:变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)和放线菌门(Actinobacteria),包括10个属(表 1).
-
采用PDA培养基、GA培养基和WA培养基从经严格表面消毒的桐乡青茎中共分离获得内生细菌25个分离株.采用细菌通用引物27F/1492R分别获得内生细菌的16S rDNA,将其序列提交至NCBI数据库GeneBank获得登录号KT766060-KT766084.经Blast序列比对,桑树内生细菌25个分离株分属于细菌域的3个类群:变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)和厚壁菌门(Firmicutes),包括12个属(表 1).
-
桑树内生细菌种群多样性分析结果表明,非培养法和培养法获得的种群分布特征差异显著.放线菌门(Actinobacteria)为非培养法特有类群,而厚壁菌门(Firmicutes)仅在培养法中得到;仅假单胞菌属(Pseudomonas)和鞘氨醇单胞菌属(Sphingomonas)两个菌属为两种方法获得的共同菌属;而在种的水平上二者没有得到共同菌种(表 1).非培养法研究结果显示,变形杆菌门(Proteobacteria)是该克隆文库中的优势类群,包含了96个克隆.文库中优势菌属为鞘氨醇单胞菌属(Sphingomonas)、Massilia和甲基杆菌属(Methylobacterium),分别占克隆文库的18.27%,16.35%和14.42% (表 1).培养法研究结果表明,变形杆菌门(Proteobacteria)亦为优势类群,包含了20个内生细菌分离株.其中肠杆菌属(Enterobacter)、芽孢杆菌属(Bacillus)和泛菌属(Pantoea)为优势菌属,分别占内生细菌总数的24.00%,12.00%和12.00% (表 1).
-
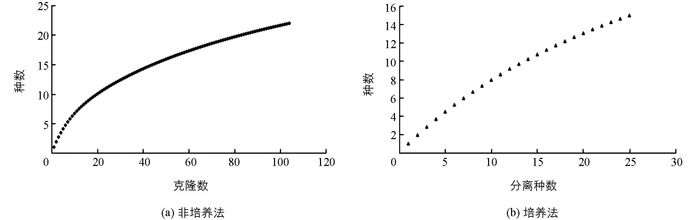
采用香农多样性指数、辛普森多样性指数、丰度、皮耶诺均匀度指数及稀疏曲线对两种方法得到的桑树内生菌多样性进行评价分析比较.桑树内生细菌16S rDNA基因文库包含111个阳性克隆,有22个分类单元,香农多样性指数为2.21,辛普森多样性指数为0.84,均匀度为0.48 (表 2),稀疏曲线趋于平缓(图 4(a)),说明该克隆文库已基本代表桑树内生细菌群落的微生物多样性.分离获得的25个内生细菌分离株,有15个分类单元,香农多样性指数为2.54,辛普森多样性指数为0.91,均匀度为0.79(表 2),稀疏曲线仍呈上升趋势(图 4(b)),说明分离获得内生菌仍较有限,培养法具有更高的多样性.
从统计分析可以看出,桑树内生菌16S rDNA克隆文库的库容基本上可反映桑树内生细菌群落的种群多样性,然而非培养法获得内生菌的丰度虽然高于培养法,但其多样性和均匀度均不及培养法,且非培养法稀疏曲线较培养法更趋于平缓,也就是说采用培养法得到的内生细菌群落复杂性高于非培养法.
-
抑菌圈法检测培养法分离获得的25株桑树内生细菌的抑菌活性,检测结果表明多达18株内生细菌分离株对靶标病原菌中的一株或者多株表现出拮抗作用,占内生细菌的72%,其中内生细菌TPY1发酵上清液对5株指示菌具有拮抗作用,具有较广谱抑菌活性(表 3).由此可见,内生拮抗菌对提高宿主植物抗病性具有一定的作用.
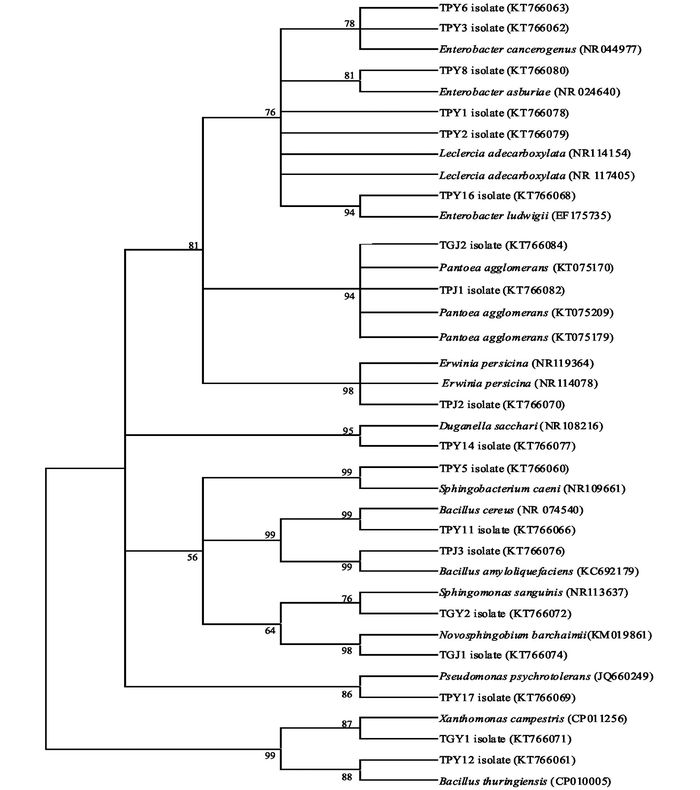
为进一步了解拮抗菌在同一宿主内的进化多样性,对18株内生拮抗细菌进行了系统发育分析.对18株拮抗内生菌的16S rDNA序列进行BLAST比对、分析,采用MEGA4. 0软件N-J法构建系统发育树(图 5).
从图 5可见,18株活性菌株在进化发育上分属11属.分离株TPY3,TPY6,TPY16为肠杆菌属(Enterobacter),其中TPY3和TPY6分离株的同源性达99%;分离株TPY1,TPY2有99%同源性,与勒克氏菌属(Leclercia)聚为一类;分离株TGJ2和TPJ1属于泛菌属(Pantoea);分离株TPJ2为欧文氏菌属(Erwinia);菌株TPY5为鞘氨醇杆菌属(Sphingobacterium);分离株TPY11和TPY12为芽孢杆菌属(Bacillus);分离株TGY2属于鞘氨醇单胞菌属(Sphingomonas);分离株TGJ1为新鞘氨醇杆菌(Novosphingobium);分离株TPY17属于假单胞菌属(Pseudomonas);分离株TGY1为黄单胞菌属(Xanthomonas).结果表明桑树内生拮抗细菌在遗传和类群上表现出较为丰富的多样性特征.
2.1. 桑树内生细菌种群分布
2.1.1. 非培养法对桑树内生细菌种群分布分析
2.1.2. 培养法对桑树内生细菌种群分布分析
2.1.3. 利用非培养法与培养法分析桑树内生细菌种群分布比较
2.2. 桑树内生菌的多样性分析
2.3. 桑树拮抗性内生细菌的初筛及系统发育分析
-
植物内生菌广泛存在于健康植物体内,是植物微生态系统的重要组成部分,对宿主的生长发育起着多种生物学作用.本研究结合非培养法和培养法对桑树(桐乡青)内生菌的种群组成进行了系统、全面的研究.为进一步了解桑树内生菌与宿主之间的关系,探究内生菌在植物体内的生命活动提供了依据,丰富了桑树内生菌资源.通常香农多样性指数介于1.5~3.5之间[25],桑树内生菌培养法与非培养法的指数分别为2.54和2.21,表明其多样性适中,与前人的研究相当,天目山山胡椒叶与茎中内生真菌香农多样性指数分别为2.63和1.83[26],乔松松针和茎内生真菌的多样性指数分别为2.48和2.23[27].
非培养法和培养方法对桐乡青内生细菌种群组成差异较大,仅有两个共同菌属,且有不同的优势种群.通过构建文库得到的细菌菌落丰富度高于分离培养方法,然而多样性指数显示培养法得到的内生菌多样性要高于非培养法,该结果表明尽管传统分离培养得到的菌株数较少,但其组成较为复杂,结合稀疏曲线可知,利用分离培养法对桑树内生细菌多样性的研究具有更高的潜力. Impullitti等人[24]对大豆内生真菌多样性研究及Mariana等人[28]对水果中内生细菌多样性研究,均得到培养法多样性指数高于非培养法.其原因可能是非培养法存在的限制因素:首先,总基因组提取需大量的植物组织,会造成总DNA中糖类、盐等杂质对后续的PCR扩增造成干扰[29];其次,总基因组在进行特异性扩增时存在PCR偏好性[24, 30];第三,某些菌株在植物内的浓度过小,非培养法无法检测[28-29];第四,本研究所构建的克隆文库只包含了16S rDNA的部分片段,ARDRA分型准确性有所下降[22];最后,由于实验条件限制,木质部无法被液氮速冻研磨,本研究非培养法仅取桐乡青韧皮部进行试验,可能会造成其多样性下降.虽然非培养法存在上述局限性,但传统分离方法亦存在菌株对培养基的选择性、不可培养菌株的存在、某些菌株生长缓慢竞争力差被生长迅速的菌株淘汰等技术问题.有多个研究报道表明,非培养法与培养法得到的内生菌种结构不一致甚至互补[29-32].因此非培养方法和培养方法结合使用可更有效、全面地研究植物内生菌的多样性,更有利于探索内生菌与宿主之间的作用.
本研究从25个分离株中共筛选得到18株对一株或多株病原菌具有抑制作用的桑树拮抗性内生细菌,占菌株总数的72%.其中有些菌株表现出较广的抑菌谱及较强抑菌活性,具有作为生物防治菌的潜能.以上结果为进一步利用内生生防菌资源,探索对植物病害的安全有效的生物防治措施提供了材料及依据.但拮抗菌的生物防治效果如何,尚需测定其在桑树体内的定殖力、持久性和回接后对桑树生长的影响,以及通过防病效果的田间试验等加以证实,也将是下一步研究的重点.



 下载:
下载: