-
植物在长期进化过程中,会逐步形成对自身有益、适宜自身生长发育的一系列调节机制,转录因子在这些调控过程中具有重要的作用.转录因子也称为反式作用因子,能够与真核基因启动子区域中顺式作用元件特异性结合,并激活或抑制基因的转录[1]. WRKY作为一种重要的锌指蛋白类转录因子,近年来研究的较为广泛.其编码基因最先在甘薯[2]中被克隆到,随后约在20多种植物中证实存在WRKY蛋白[3]. WRKY转录因子最显著的特征是含有一个由60个氨基酸组成的多肽,这个多肽N端至少含有一个WRKY的核心结构域WRKYGQK,C端含有一个非典型的锌指结构[4].根据WRKY锌指结构的特征及WRKY结构域的数量,将WRKY蛋白分为3类:第Ⅰ类含有1个WRKY结构域,锌指结构为CX4-5CX22-23HX1H;第Ⅱ类含有2个WRKY结构域,其锌指结构是CX4-5CX22-23HX1H,与第Ⅰ类的结构相同;第Ⅲ类与第Ⅰ类同样含有1个WRKY结构域,但是锌指结构不同,为CX7CX23HX1C[4].大多数的WRKY转录因子属于第Ⅱ类,而第Ⅲ类WRKY转录因子一般只存在于高等植物中,并且能响应多种生物胁迫[5].
近年来,研究发现WRKY基因广泛存在于各个物种中:拟南芥中有72个[6],水稻中有102个,大豆中有197个[7],番茄中有81个[8].对这些WRKY基因的表达研究表明,它不仅能在植物受到生物胁迫和非生物胁迫时诱导表达,还能受到外源施加激素的诱导表达.现有的研究表明,WRKY基因在植物生物胁迫或非生物胁迫过程中具有很重要的作用.在生物胁迫方面,超表达辣椒的WRKY基因CaWRKY40能增强烟草对茄科雷尔氏菌的抵抗力[9];拟南芥中超表达AtWRKY38和AtWRKY62后,植株对丁香假单胞菌的侵染更加敏感[10];同样,将辣椒的一个WRKY基因CaWRKY27在烟草里面超量表达,提高了烟草抗青枯病的能力[11].在非生物胁迫方面,拟南芥的AtWRKY57通过提高拟南芥ABA水平来增强其对干旱的耐受性[12];小麦中的TaWRKY44在烟草中超量表达提高了烟草抗多种非生物逆境的能力[13];棉花中的GhWRKY34基因在拟南芥中超量表达提高了其抗盐碱的能力[14].此外,有些WRKY基因既在生物逆境中又在非生物逆境中有作用,如棉花的GhWRKY25可以调节SA和JA信号途径来降低植物对病原菌的抵抗能力和抗旱能力[15].
番茄(Solanum lycopersicum)是世界性的重要的蔬菜作物,全球番茄产量达到1.6亿吨,其中超过30%产自我国(FAO,2015),在我国农业产业中具有重要的地位.随着全球气候变化的加剧,非生物逆境越来越成为农作物产量和品质提高的严重制约因素,也影响着植物的进化和分布[16-17].利用野生番茄种质资源探索植物抗逆分子机制,可为创造抗逆新品种奠定理论基础及提供实践价值.普通栽培番茄(S.lycopersicum)对干旱和高盐较为敏感[18],而其近缘野生种潘那利番茄(S. pennellii)起源于秘鲁安第斯山脉,可在干旱和半干旱环境中生存,对高盐和干旱不敏感[19].分析抗旱野生种和普通栽培种在干旱响应基因中的差异,通过转基因的方法将野生种相关基因转化到普通栽培番茄种中,是提高栽培番茄抗逆性的一种非常有效的方法.本研究分别从抗旱野生种番茄和栽培番茄中分离出一个WRKY基因,分析了它们的表达特性,并构建了超量表达载体,成功转化进普通栽培番茄M82中,为今后深入研究该基因的抗逆功能和提高番茄的抗逆性奠定基础.
全文HTML
-
基于前期的干旱基因芯片结果,我们分离得到1个在普通番茄栽培品种S. lycopersicum cv. M82和野生番茄品种S. pennellii LA0716在干旱处理下有表达差异的基因WRKY41(Unigene SGN-U212725,https://solgenomics.net).根据Unigene序列,利用Primer Premier 5.0设计引物,用RT-PCR得到基因的全长ORF.在番茄五叶一心时,采集番茄从上到下第三片功能叶,用液氮保存,研磨. RNA采用Trizol(Invitrogen,USA)一步法提取.约1 μg总RNA样品经1U的DNaseI(Fermentas,USA)37 ℃孵育30 min后,加入1 μl EDTA (50 mM),于65 ℃温育10 min.取各样品总RNA,以Oligo-d(T)为引物,用AMV反转录酶进行反转录,参照PrimeScript® 1st Strand cDNA Synthesis Kit(TaKaRa)方法进行.扩增基因WRKY41引物对为:WRKY41正向引物5′-TTAGCAACTTTCCCCCTTCA-3;WRKY41反向引物5′-TGAATTCATCGACGTTACAATTT-3′.利用PrimerSTAR HS高保真酶(Takara,大连)按说明书进行PCR扩增. PCR产物经0.8%的琼脂糖凝胶电泳后,回收特异性条带.将回收后的片段与pEASY-Blunt(TransGen)克隆载体进行连接.热击转化大肠杆菌DH5α,筛选阳性克隆进行测序验证.
利用WRKY41氨基酸序列对NCBI的蛋白质序列数据库进行BLAST,得到序列和其他物种相似性较高的氨基酸序列,然后用ClustalW进行多序列比对.利用SGN(https://solgenomics.net)数据库找出番茄所有的WRKY基因,利用MEGA5.1构建Neighbor-Joining系统进化树.
-
番茄常规品种M82和野生番茄品种LA0716用于表达分析,常规栽培管理,于2014年5-6月栽培在西南大学蔬菜实验室温室.在植株开花结果时,收集地上部分的茎(ST)、幼叶(YL)、成熟叶(ML)、老叶(OL)花(FL)和果实(FR),将材料立即放入液氮中速冻,并保存于-80 ℃超低温冰箱备用,用于分析SlWRKY41的组织表达特性.当植株长至五叶一心时取样,用于各种调控因子下的表达分析.各种处理具体如下:干旱脱水(DH)胁迫的是将离体植株放在滤纸上,环境条件为70%的相对湿度,室温25 ℃;ABA(脱落酸)、SA(水杨酸)、GA(赤霉素)处理、Eth(乙烯)和氧化处理,采取直接喷施100 μmol/L ABA、100 μmol/L SA、100 μmol/L GA、1 mM乙烯利(乙烯Eth释放剂)和100 μmol/L的甲基紫精MV(含0.2% Tween-20),过量喷施,直到叶片滴水为止.每次重复分别采集三棵植株的三片叶,在设计好的时间点收集样品按上述方法保存样品备用.
-
分别提取番茄不同组织器官和各种处理材料的总RNA,并进行反转录,方法如1.1所述.根据Primer 3(http://primer3.ut.ee/)设计半定量和实时定量引物.在WRKY41开放读码框内设计基因引物WRKY41(Q):正向引物5′-ATTCCCTCCTGCACCACTAC-3,反向引物5′-TAATCCACCCCTTCTCCACC-3,扩增长度213 bp.以番茄β-Actin(SGN-U580609) 作为内参基因校正,正向引物5′-ATGGCAGACGGAGAGGATATTCA-3,反向引物5′-GCCTTTGCAATCCACATCTGCTG-3.半定量首先用β-Actin进行PCR校正各个组织的cDNA(27个循环),校正一致后,通过目的基因的PCR扩增,通过电泳图来确定基因在各个组织中的表达情况.
实时定量PCR按照SYBR® Premix Ex TaqTM操作说明,在Bio-Rad CFX96荧光定量PCR仪上进行,检测目的基因的相对表达量.荧光定量PCR反应体系为10 μL:2×SsoFast EvaGreen supermix (Bio-Rad) 5 μL,正向反向引物各0.5 μL,cDNA模板2 μL,超纯水2 μL. PCR反应程序为95 ℃预变性3 min,95 ℃变性10 s,58 ℃退火30 s,65 ℃延伸5 s,39次循环.每个试验设置3次重复.为了消除光周期对基因表达的影响,在每个时间点分别取未处理和处理的样品,每个时间点的表达量以未处理的表达量为1,处理之后和未处理相比计算得到相对表达量.
-
将1.1得到的SpWRKY41经过克隆质粒用限制性内切酶SalI和KpnI酶切.电泳切胶回收得到目的片段,用T4连接酶连接到用XhoI和KpnI酶切双元载体pBI121的载体框架上.得到的载体经过PCR验证后,抽提好质粒电击法转到农杆菌C58感受态细胞,保存用于番茄的遗传转化.利用农杆菌介导法转化到番茄M82中,具体的操作流程参考欧阳波的方法[20].得到转基因植株后,利用35S(5′-TTCGCAAGACCCTTCCTCTA-3) 加上基因反向引物进行PCR验证.
1.1. SlWRKY41基因的克隆和序列分析
1.2. SlWRKY41表达模式分析
1.3. 半定量和实时荧光定量PCR
1.4. 转基因植株的获得及鉴定
-
基于前期干旱芯片分析结果,WRKY41(SGN-U212725) 受到干旱诱导并且在番茄野生种和栽培种中有表达差异.根据其基因片段设计引物WRKY41正向和反向引物,以普通番茄M82和野生种番茄潘那利叶片的cDNA为模板进行PCR扩增,扩增后的片段回收,然后连接到克隆载体上,在板上挑取单克隆进行PCR鉴定,菌落PCR结果得到一条大约长1 271 bp的特异条带(图 1).测序结果表明,基因的全长ORF为1011bp,编码336个氨基酸.通过与NCBI数据库进行BLAST后,该蛋白与其他物种中的这类蛋白都含有一个保守的WRKY结构域,并且具有很高的保守性(图 2),如马铃薯和烟草的相似性最高,分别达到了90.48%和53.27%,另外和葡萄、可可、咖啡、丹参、菊花、桉树、棉花、杨树、蓖麻、苹果的相似性也达了30%左右.
普通栽培种中克隆到的SlWRKY41和野生种中的SpWRKY41的相似性达到97.62%,只有5个氨基酸的差异(图 2).具体的差异为:第87位在M82中的Ser丝氨酸而在潘那利(P)中是Asn天冬酰胺,第244位点天冬氨酸Asp(M)突变成谷氨酸Glu(P),第275位点谷氨酸Glu(M)突变成丙氨酸Ala(P),第281位点丙氨酸Ala(M)突变成苏氨酸Thr(P),第283位点脯氨酸Pro(M)突变成谷氨酰胺Gln(P);其中在281和283位点的两个疏水性氨基酸(Ala和Pro)突变成了亲水性的氨基酸(Thr和Gln).这些突变可能会造成氨基酸结构和功能的差异,从而导致在番茄栽培种和野生种中WRKY41基因功能的差异.
-
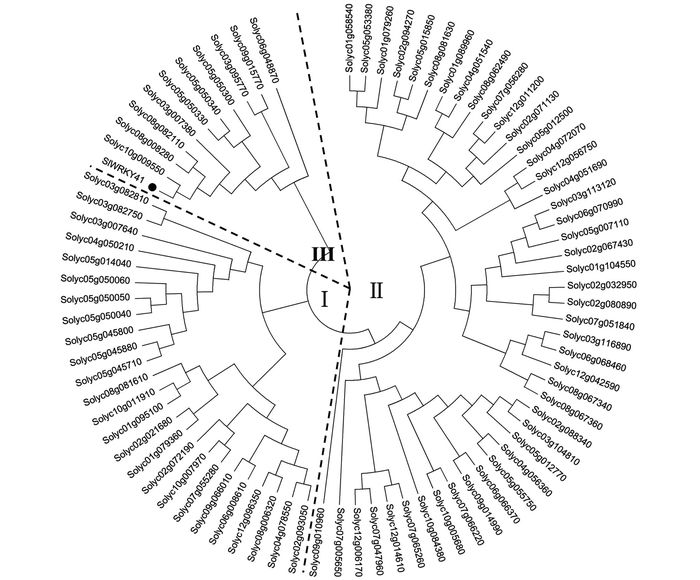
通过生物信息学的方法,从番茄的基因组中分离出来了81个WRKY基因.这些基因编码的蛋白最长的达到739个氨基酸(Solyc07g066220.2.1),最短的只有131个氨基酸(Solyc02g094270.1.1),平均氨基酸长度为354个.系统进化树分析表明,发现番茄中的WRKY蛋白可分为三大类,第Ⅰ类含25个WRKY蛋白,第Ⅱ类有45个WRKY. WRKY41属于第Ⅲ类,这类蛋白包含有11个WRKY蛋白,他们都只含有一个WRKY结构域,并且其锌指结构为CX7CX23-24HX1C或者CX4CX23HX1C.
-
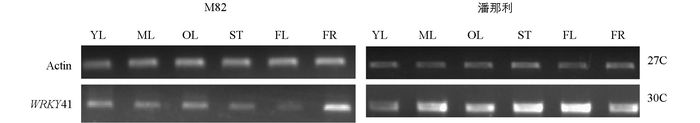
以番茄Actin为内参,半定量RT-PCR分析了WRKY41基因在普通栽培番茄品种M82和野生抗旱番茄潘那利中的组织表达特性.从图 4可以看出,在M82中,WRKY41在叶片(包括老叶、成熟叶和嫩叶)和果实中表达量比较高,但是在潘那利中表达模式和在M82中有差异,它主要在成熟叶、茎和花中表达量高.这些表达模式的差异可能是由于品种的差异造成的.
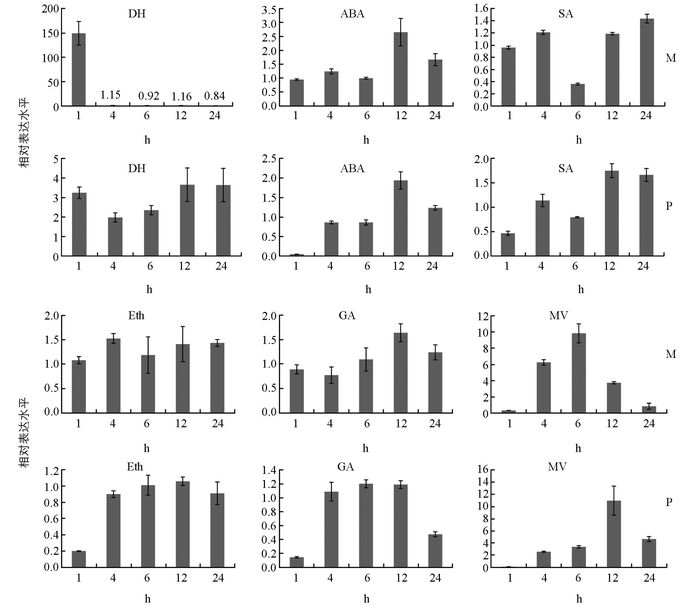
为了探索WRKY41在多种逆境下和调节因子处理下的表达模式,我们分别做了一系列处理和不同时间点分析,这些处理包括脱水处理(DH)、ABA、SA、乙烯利(Eth)、GA和氧化逆境(甲基紫精MV)处理.从图 5中可以看出,在脱水处理M82植株的情况下,在1 h时WRKY41的表达迅速提高,表达量比未处理的植株提高了近150倍,但在4h后基因表达迅速下降到原来未经诱导之前的水平,无明显的上调表达趋势;在野生抗旱番茄品种潘那利中,WRKY41一直处于比较高的上调表达水平;这些结果说明,无论是野生种还是普通栽培品种中,在脱水的情况下,WRKY41均能够被诱导表达,并且在潘那利中能够稳定地提高表达.在ABA处理下,在M82和潘那利中,WRKY41都呈现上调表达趋势,表达模式基本相同,都在12 h时表达量达到最高.在SA处理下,在M82和潘那利中,WRKY41基因的表达模式也基本一致,呈负调控表达趋势,在M82中6 h时基因显著下调表达(<2倍),但在潘那利处理1 h时,基因达到了显著的下调表达,说明基因在潘那利中对SA响应比较快.乙烯利和GA处理M82时,WRKY41基因的表达没有显著的差异,但是在处理潘那利的过程中,在刚开始处理1 h后,基因迅速的被抑制表达(>5倍),而且在GA处理24 h后,基因还是处于被抑制表达的状态,这说明WRKY41在潘那利中对乙烯和GA能够迅速响应,但是在M82中无明显变化.在氧化逆境的情况下,基因都被诱导表达,只是在M82中,基因在6 h时表达量最高,在潘那利中12 h时表达量最高.上述结果表明,WRKY41能被多种逆境和调节因子的诱导表达,而且它在普通栽培番茄M82和野生种番茄潘那利中呈现不同的表达模式.
-
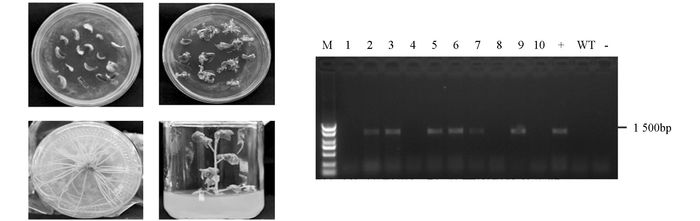
将潘那利番茄的SpWRKY41构建了超量表达载体,通过农杆菌介导法转化番茄栽培种M82. 图 6为番茄转化的几个阶段.
提取DNA后,以未经转化的植株为对照,利用35S加WRKY41基因反向引物对转基因植株进行PCR鉴定,经1%琼脂糖凝胶电泳分析,得到PCR扩增出1 500 bp左右的目标条带(图 6),10株番茄抗性组培苗中有6株获得了预期大小的目标条带,表明目的基因SpWRKY41已整合到番茄基因组中.
2.1. 番茄WRKY41基因全长序列的克隆
2.2. 番茄WRKY41编码进化树分析
2.3. WRKY41的表达特性分析
2.4. 转基因植株的获得及鉴定
-
WRKY转录因子广泛存在于植物中,大多数是受外界环境的激发而诱导表达,参与植物生物与非生物胁迫应答反应、植物衰老以及果实发育等一系列生理活动[21].在本研究中,我们从普通栽培番茄品种M82和野生耐旱番茄潘那利中分别克隆得到了一个WRKY基因WRKY41,命名为SlWRKY41和SpWRKY41(图 1),这个基因具有典型的WRKY结构域和CX7CX23HX1C锌指结构域,这和前人的报道一致[4].进一步分析发现,来自栽培种和野生种番茄的WRKY在核苷酸序列上是不一样的,核苷酸序列的变异造成5个氨基酸的差异(图 2),这些氨基酸的差异可能会导致蛋白与蛋白之间的相互作用,从而导致不同近缘种之间的功能差异[22],这些变异可能是植物在长期的进化过程中造成的.
进化分析表明,WRKY41属于第Ⅲ类WRKY转录因子,这类转录因子是高等植物所特有的[23],响应多种逆境和调节因子.有研究证明,拟南芥基因AtWRKY18经过2 mmol/L SA处理3h后能快速被诱导表达,此时mRNA积累达到高峰[24].王彦华等研究表明,白菜中获得的WRKY1基因的cDNA片段,在2 mmol/L SA处理4h后被快速诱导表达[25]. Ryu等在研究中发现水稻中的OsWRKY45和OsWRKY62在SA处理12 h后表达量增加[26];厚叶悬蒴苣苔的WRKY基因BcWRKY1在2 mmol/L的SA处理8 h后才被诱导表达,但在24 h后,表达量降低.同样,BcWRKY1可在JA和ABA处理后被诱导表达[27];喷洒乙烯利8 h后葡萄的VvWRKY1表达量显著增高,而经过SA处理后2 h才开始表达,但在4 h和8 h后达到最大值[28].我们从前期干旱芯片中分离出来的这个WRKY基因,经过验证确实受到干旱脱水的诱导表达,并且在栽培番茄品种M82和野生番茄潘那利中表达模式存在差异(图 5),在M82中,基因处理1 h后表达迅速提高,但在1 h后一直到24 h,基因表达量降低或很难检测到其表达,可能是干旱脱水后,M82植株迅速萎焉,基因不表达或表达量非常低,但是在潘那利中,WRKY41一直稳定上调表达,因为潘那利在干旱脱水的情况下,不会迅速萎焉,推测潘那利之所以抗旱,可能是因为很多抗旱相关基因一直稳定表达所造成的.植物在非生物逆境发生时,自身会生成超氧自由基,并产生氧化逆境,在氧化逆境处理中,WRKY41基因在两个番茄材料中都被诱导表达.同样,在ABA诱导下,WRKY41在两个番茄材料中也被诱导表达,表达模式基本一致.但在SA、Eth和GA的处理下,潘那利番茄中的WRKY41在1 h时便迅速响应而下调表达;而在M82中,该基因在Eth和GA诱导下,无差异表达,在SA诱导下的6 h时才响应并下调表达,这些都说明在潘那利中,WRKY41能迅速响应表达来适应不利的环境.这说明在野生种中,抗逆基因是通过迅速响应不利环境信号来表达的,这个可能在植物抗逆中有着重要的意义.
栽培种番茄经过长期的环境条件以及人工的选择,导致很多重要的抗旱性状遗传基因的等位变异和丢失,造成育种的遗传资源匮乏,也容易出现“平台效应”.有些番茄的野生种由于长期生长在高山、沙漠等极端恶劣的环境中,经过环境的变化与自身的适应性,会使其携带一些抗性基因,因此,利用野生抗逆基因及优异的等位变异,是加速番茄抗逆育种的关键.采用传统的有性杂交育种方式,可以转育野生种的抗性基因到栽培番茄中,但由于连锁累赘问题,通常也会带入一些野生种的不利性状.目前,通过转基因的方法提高植物的抗逆性是最有效的方法之一,我们可以通过分析野生种和栽培种之间重要基因的结构和表达差异,再将来自野生种的基因转化到普通栽培番茄,这将是改良番茄植株的一个有效的方法,本研究已经将来自于潘那利的基因SpWRKY41通过转基因的方法转化到M82中,后期将对其进行功能分析,确定基因功能,改良番茄抗旱性.



 下载:
下载: