-
近些年来,N-二苯基亚甲基甘氨酸酯衍生物的合成备受关注,因为该类化合物是最重要的希夫碱之一,可作为各种手性的天然或非天然氨基酸的前体,用于具有生物活性的化合物的合成[1-2].目前文献报道的方法大多是关于非手性的N-二苯基亚甲基甘氨酸酯的合成[3],含手性辅基的N-二苯基亚甲基甘氨酸酯的合成方法却鲜有报道.手性辅基作为一种高效的构建不对称中心的手段,被广泛地用于具有生物活性的天然产物和药物分子的合成.例如,在手性辅基不对称诱导的作用下,通过不对称烷基化反应[4]和加成反应等[5],N-二苯基亚甲基甘氨酸酯衍生物的α-氢可以有选择性地被不同的基团取代,合成高光学纯度的异构体,再经过水解等步骤,可以制备各种不同的具有手性的天然或非天然氨基酸[6].因此,发展一条手性N-二苯基亚甲基甘氨酸酯的高效合成路线显得十分重要.
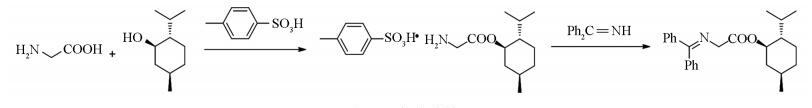
本文报道了一种(-)-N-二苯基亚甲基甘氨酸薄荷醇酯的简捷高效的制备方法,首先由甘氨酸和薄荷醇在对甲苯磺酸催化下发生酯化反应,用得到的甘氨酸薄荷醇酯磺酸盐,直接和二苯甲酮亚胺在室温下发生缩合反应,能以较高的产率得到目标产物,其合成路线见图 1.
全文HTML
-
试剂:甘氨酸(BR)、对甲苯磺酸(一水)(AR),成都市科龙化工试剂厂;(-)-薄荷醇(AR)、二苯甲酮亚胺(AR),Adamas Reagent Co.,Ltd.;甲苯(AR)、二氯甲烷(AR),重庆川东化工(集团)有限公司化学试剂厂;薄层层析硅胶300~400目(AR),青岛海洋化工集团公司;其余试剂均为市售分析纯产品.
仪器:Bruker Avance 600型核磁共振仪;RE-52A型旋转蒸发器(上海亚荣生化仪器厂);85-2型恒温磁力搅拌器(上海司乐仪器有限公司);C型玻璃仪器气流烘干器(长城科工贸有限公司);101A-2E型电热鼓风干燥箱(上海实验仪器厂有限公司);DF-101S集热式恒温加热磁力搅拌器(长城科工贸有限公司);SHB-IIIG型水泵(长城科工贸有限公司);DLSB-5L/20型低温冷却液循环泵(巩义市予华仪器有限责任公司).
-
取2 g(26.6 mmol)甘氨酸、4.92 g(32.0 mmol)(-)-薄荷醇和6 g(32.0 mmol)对甲苯磺酸(一水)于100 mL单口圆底烧瓶中,加入40 mL甲苯,安装分水器和回流冷凝管,使用油浴加热回流,反应直至收集到接近理论量的水.反应结束后,冷却并抽滤,滤液旋蒸除去溶剂得到油状物即为磺酸盐粗品,无需提纯,直接进行下一步.
-
取本实验所制备的甘氨酸薄荷醇酯对甲苯磺酸盐和4 mL(26.6 mmol)二苯甲酮亚胺于100 mL单口圆底烧瓶中,加入二氯甲烷并搅拌溶解.在室温下反应0.5 h后抽滤,滤液旋蒸除去溶剂得到(-)-N-二苯基亚甲基甘氨酸薄荷醇酯粗品.使用柱层析提纯粗品得到(-)-N-二苯基亚甲基甘氨酸薄荷醇酯纯品(硅胶,V(石油醚):V(乙酸乙酯)=20:1).
(-)-N-二苯基亚甲基甘氨酸薄荷醇酯的核磁数据为:1H NMR (600 MHz,CDCl3)δ7.66 (d,J=7.5 Hz,2H),7.48~7.43 (m,3H),7.40 (t,J=7.3 Hz,1H),7.33 (t,J=7.6 Hz,2H),7.20~7.16 (m,2H),4.76 (td,J=10.9,4.4 Hz,1H),4.19 (s,2H),2.04~1.98 (m,1H),1.85 (dtd,J=13.8,6.9,2.4 Hz,1H),1.70~1.65 (m,2H),1.65 (s,1H),1.54~1.44 (m,1H),1.39~1.32 (m,1H),1.10~1.01 (m,1H),0.96 (dd,J=23.3,11.9 Hz,1H),0.90 (d,J=6.5 Hz,3H),0.87 (d,J=7.0 Hz,3H),0.75 (d,J=6.9 Hz,3H).13C NMR (151 MHz,CDCl3)δ 171.72,170.10,139.39,136.13,130.37,128.76,128.61,128.02,127.71,74.67,55.91,47.12,40.89,34.27,31.39,26.23,23.52,22.00,20.73,16.40.
1.1. 主要试剂和仪器
1.2. 实验步骤
1.2.1. 甘氨酸薄荷醇酯对甲苯磺酸盐的合成
1.2.2. (-)-N-二苯基亚甲基甘氨酸薄荷醇酯的合成
-
目前,文献报道的合成方法主要有氯乙酸酯和二苯甲酮亚胺缩合法[7],以及甘氨酸酯和二苯甲酮缩合法[8]等.第一种方法需要惰性气体保护,反应温度高,耗时长,而且二苯甲酮亚胺很不稳定,容易分解,不易操作;第二种方法需要用到三氟化硼乙醚、三氯化铁等催化剂,反应条件苛刻,试剂具有毒性,对环境污染大,且需要氨基的保护和脱保护[8],步骤繁琐.因此开发廉价、简便、高效的合成方法意义重大.本研究采用甘氨酸、薄荷醇和对甲苯磺酸反应形成甘氨酸酯的磺酸盐,再和二苯甲酮亚胺在室温下反应的方法.与第二种方法相比,对甲苯磺酸作为一种便宜、易得的固体有机酸,可有效地催化薄荷醇和甘氨酸的直接酯化反应,生成的磺酸盐作为稳定的中间体可直接进行下一步反应,从而使该合成路线更为高效和快捷.
-
为提高反应产率,本实验对甘氨酸、薄荷醇和二苯甲酮亚胺的投料比例进行了研究[9-10],结果见表 1.
由表 1可见第一步反应中薄荷醇为1.2 mmol时较1.0 mmol时产率有大幅提升,其过量有利于酯化反应进行彻底.而对于第二步反应,降低二苯甲酮亚胺用量导致产率相应下降,而提高其用量并没有致使产率大幅提高,1.0 mmol二苯甲酮亚胺用量下的产率亦较为可观.因此,为节省原料、简化后处理,采用n(二苯甲酮亚胺):n(甘氨酸):n(薄荷醇)=1:1:1.2为最佳条件.
-
本实验对甘氨酸薄荷醇酯对甲苯磺酸盐和二苯甲酮亚胺反应的温度进行了考察[11],结果见表 2.
由实验可见,当温度高于室温时,反应产率有所下降,这是由于二苯甲酮亚胺性质不稳定,在较高温度下容易分解.在此温度下,需要提高二苯甲酮亚胺用量,才能到达较高的产率.然而此法并不经济,所以该反应不宜在高温下进行.此外,在低温下能够有效抑制二苯甲酮亚胺的分解,但0 ℃时反应时间需延长,产率却没有大幅提升.因此,采用室温为最适宜的反应温度.
-
本实验对甘氨酸薄荷醇酯对甲苯磺酸盐和二苯甲酮亚胺反应使用的溶剂进行了筛选[12],结果见表 3.
二苯甲酮亚胺在含水的溶剂中更易分解,本实验对经过无水处理的二氯甲烷作为溶剂时的反应效果进行了分析.结果发现,在室温下使用经过无水处理的二氯甲烷作为反应溶剂和没有经过无水处理的二氯甲烷作为反应溶剂,其效率相差无几.因而,在考虑简化操作的因素下,使用普通分析纯的反应溶剂更为简便.此外,表 3显示使用甲苯和四氢呋喃作为反应溶剂,也可得到较高的反应产率.但甲苯相比二氯甲烷沸点较高,不宜蒸发除去,四氢呋喃易产生过氧化物,有爆炸危险,因此在考虑安全和操作的层面上采用二氯甲烷为反应溶剂更为适宜.
2.1. 投料比
2.2. 反应温度的筛选
2.3. 溶剂的筛选
-
本研究提出的方法能快速高效地合成(-)-N-二苯基亚甲基甘氨酸薄荷醇酯;在投料比为n(二苯甲酮亚胺):n(甘氨酸):n(薄荷醇)=1:1:1.2、反应温度为室温、溶剂为二氯甲烷的最佳条件下,可以得到高产率的(-)-N-二苯基亚甲基甘氨酸薄荷醇酯.此方法不需要氮气保护,不需要保护氨基,缩合无需使用催化剂,反应条件温和,操作简单,绿色环保,经济节能.



 下载:
下载: