-
宁夏酿酒葡萄主栽区为贺兰山东麓的狭长地带,其独特地理气候优势为酿酒葡萄生长提供了一个理想的环境,但葡萄病毒病尤其是葡萄卷叶病危害严重,降低了酿酒葡萄的产量和品质,也影响了宁夏酿酒葡萄产业的发展.与葡萄卷叶病相关的病原为葡萄卷叶伴随病毒(Grapevine leafroll-associated virus,GLRaVs),目前已报道的GLRaVs至少有5种,分别是GLRaV-1,GLRaV-2,GLRaV-3,GLRaV-4 (包括GLRaV-4的变种GLRaV-5,GLRaV-6,GLRaV-9,GLRaV-De,GLRaV-Pr和GLRaV-Car)和GLRaV-7[1-3].在国内各葡萄产区,感染葡萄卷叶病的葡萄园中GLRaV-1和GLRaV-3发生最为普遍,报道也最多[4-7].
2016年,吕苗苗等[7]对宁夏各种植区的8个主栽酿酒葡萄品种田间自然发病情况进行了调查,发现树龄8年以上的葡萄园GLRaVs的感染率均在50%以上,个别品种如“蛇龙珠”和“黑比诺”的发病率高达85.1%和52.7%. 2009年,顾沛雯[8]通过对CP基因的克隆和序列比对,研究GLRaV-3株系间的差异. 2018年,吕苗苗等[9]通过CP,RdRp和HSP70基因检测宁夏酿酒葡萄GLRaVs种类,检出率最高的为GLRaV-1和GLRaV-3.目前,宁夏地区已报道的GLRaVs检出种类主要为GLRaV-1,GLRaV-3和GLRaV-5[7-10].
单链构象多态性(single-strand conformation polymorphism,SSCP)技术是一种有效检测已知基因点突变或未知变异分析的方法[11-12]. Turturo[13]利用SSCP技术对14个国家的GLRaV-3的变异进行了综合分析,发现不同分离物间存在分子差异.目前,国内对GLRaVs的研究主要集中在检测技术的优化[8, 14-16]和无毒苗木的培育[17-19]等方面,而对GLRaVs的遗传变异研究较少.
本研究在已有研究基础上,对宁夏两个酿酒葡萄主产区的15份GLRaVs样本展开研究,以GLRaV-1和GLRaV-3分离物为研究对象,利用SSCP技术进行其遗传变异分析,初步揭示宁夏GLRaV-1和GLRaV-3种群的分子特性,本研究将有助于宁夏地区酿酒葡萄品种GLRaVs流行学调查和分子诊断分析,为该病害防治提供理论依据.
全文HTML
-
供试材料均采自宁夏银川和永宁地区,分别为银川地区志辉源石酒庄、新牛酒庄和广夏三基地;永宁地区立兰酒庄、新惠彬酒庄、玉泉营农垦东大滩和玉泉营农垦南大滩.酿酒葡萄品种为赤霞珠(Cabernet Sauvignon)、蛇龙珠(Cabernet Gernischt)、黑比诺(Pinot Noir)、霞多丽(Chardonnay)、品丽珠(Cabernet Franc)、西拉(Shiraz)和梅鹿辄(Merlot).酿酒葡萄病株样品采集情况见表 1.
-
依据文献资料[5, 20-21],登录NCBI(www.ncbi.nlm.nih.gov)设计GLRaV-1和GLRaV-3的特异性引物(表 2),引物由上海生工生物工程股份有限公司合成.
-
葡萄叶柄韧皮部组织100 mg,加入液氮迅速研磨至粉末状,并迅速转移至1.5 mL离心管中,按照提取植物总RNA试剂盒(美国OMEGA公司)使用说明书进行总RNA的提取.
-
将提取的总RNA,利用转录试剂盒(全式金公司)反转录成cDNA,反应体系为20 μL:总RNA 4 μL,OligDT18 Primer 1 μL,RNase-free Water 3 μL,65 ℃温浴5 min,冰浴2 min;加入2×TSReaction Mix 10 μL,TransScriptTMRT 1 μL,gDNA Remover 1 μL,轻轻混匀,42 ℃孵育30 min,85 ℃加热5 s,失活TransScriptTMRT和gDNA Remover,即得到所需的cDNA.
利用GLRaV-1和GLRaV-3特异性引物对15个样品进行病毒检测. PCR扩增反应体系为25 μL:总cDNA 1.5 μL,正、反向引物各1 μL,Maxtaq酶12.5 μL,RNase-free Water 9 μL;PCR扩增条件:94 ℃ 3 min;94 ℃ 1 min,55 ℃ 45 s,72 ℃ 1 min,35个循环;72 ℃ 10 min;4 ℃ ∞.用1%琼脂糖凝胶对PCR产物进行电泳检测,阳性样品PCR产物备用.
-
配制30%聚丙烯酰胺(交联度29:1),10%过磷酸铵(APS)溶液,PCR-SSCP变性缓冲液,10× TBE Buffer,固定液,0.2%银染液和显色液.
-
按比例配制制备胶:30%聚丙烯酰胺8 mL,5×TBE 6 mL,10% APS 250 μL,TEMED 35 μL,ddH2O补足30 mL,置4 ℃冰箱预冷30 min.阳性样品PCR产物4 μL加入至6 μL变性缓冲液中,混合均匀后95 ℃水浴变性10 min后,立即冰浴5 min,迅速上样,4 ℃条件下160 V电泳45 min.
-
将胶板割下,清水冲洗;加入固定液固定7 min,清水轻轻冲掉固定液;再用银染液银染10 min,清水冲洗;后用显色液显色7 min,冲洗显色液后进行观察,拍照保存.
-
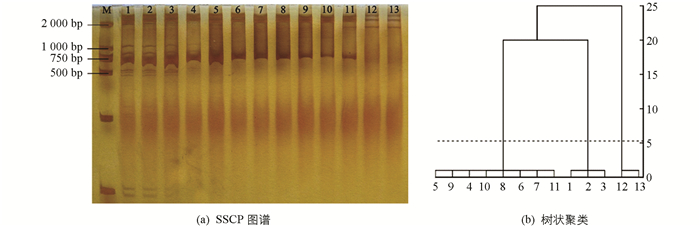
非变性聚丙烯凝胶电泳(非变性PAGE)中单链DNA空间构象的位阻实现不同条带的表达[22]. GLRaV-1和GLRaV-3阳性克隆PCR产物进行SSCP分析,利用统计软件SPSS 19.0对各样品在非变性PAGE中的条带数和迁移长度进行数据处理并构建树状聚类.
1.1. 材料
1.2. GLRaVs的分子检测
1.2.1. 引物序列
1.2.2. RNA提取方法
1.2.3. RT-PCR检测
1.3. SSCP分析
1.3.1. 溶液配制
1.3.2. SSCP检测
1.3.3. 银染过程
1.4. 聚类分析
-
按照提取RNA试剂盒(美国OMEGA公司)使用说明书进行总RNA的提取,所有提取的RNA样本凝胶电泳检测有两条清晰明亮带,分别为28S rRNA和18S rRNA;其OD 260/280值介于2.0~2.2之间,完整性和纯度较好,满足RT-PCR要求.
-
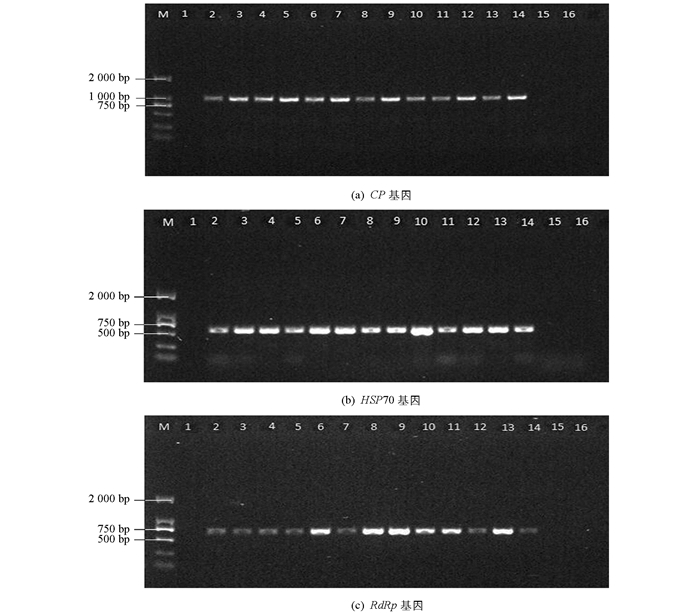
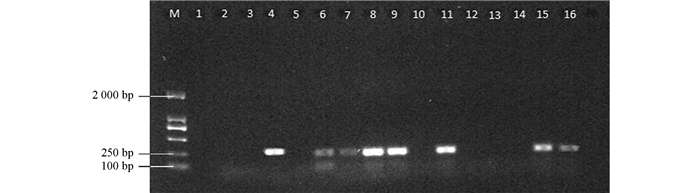
利用GLRaV-1 CP基因的序列特异性引物进行RT-PCR检测,检出8个阳性样本在230 bp位置出现亮带(图 1),与预期的GLRaV-1 CP基因序列大小相符.
-
利用GLRaV-3 CP,HSP70和RdRp基因的序列特异性引物进行RT-PCR检测,检出13个阳性样本分别在940,540和680 bp位置出现亮带(图 2),与预期的GLRaV-3 CP,HSP70和RdRp基因序列大小相符.通过GLRaV-3的CP,HSP70和RdRp基因检测发现,阳性样品的检测率一致.
-
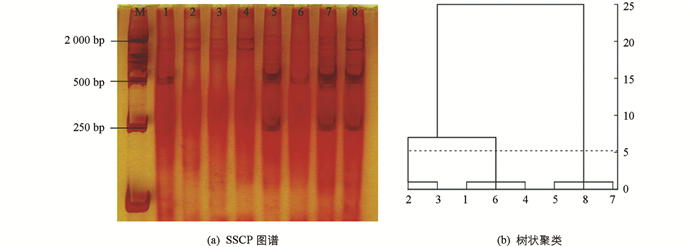
8个GLRaV-1 CP基因的阳性样本PCR产物在非变性PAGE中,实现了不同条带的表达.结合样本在SSCP图谱(图 3a)中的迁移长度进行树状聚类分析(图 3b),发现8个分离物之间有明显差异,可归为3类:分离物XHB-P,XHB-S和XHB-XL的相似性很高,带数均为4条,迁移长度为1.7 cm,归为Ⅰa类;XHB-C,DDT-S和DDT-C的相似性很高,带数均为3条,迁移长度为1.5 cm,归为Ⅰb类;LL-X和XHB-H的相似性很高,带数均为2条,迁移长度为1.0 cm,归为Ⅰc类. 8个GLRaV-1分离物分布存在明显的地域性差异.永宁地区酿酒葡萄品种蛇龙珠得到的分离物XHB-S和DDT-S分别归为Ⅰa和Ⅰc类;而酿酒葡萄品种蛇龙珠和品丽珠的分离物XHB-S和XHB-P则归为Ⅰb类,从黑比诺和霞多丽得到的分离物XHB-H和LL-X则归为Ⅰc类.
-
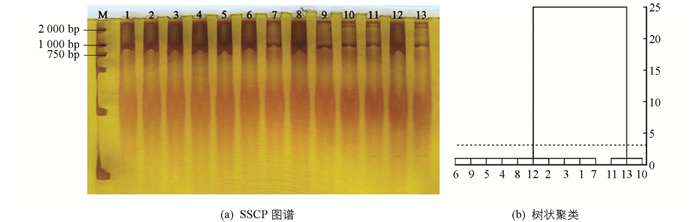
13个GLRaV-3 CP基因的阳性样本PCR产物进行SSCP分析,GLRaV-3 CP基因的SSCP图谱表现为2种带型(图 4a),结合在图谱中迁移的长度进行树状聚类分析(图 4b),发现13个分离物之间有明显差异,可归为2类:GLRaV-3 CP基因分离物LL-C,ZH-C,XN-S,DDT-S,LL-X,GX-P,XHB-P,GX-XL,NDT-M和GX-M的相似性较高,带数为1条,迁移长度为2.3 cm,归为Ⅱa类;分离物DDT-C,XHB-S和XHB-H的相似性较高,带数为2条,迁移长度为1.8 cm,归为Ⅱb类.
-
对13个GLRaV-3 HSP70基因阳性样本PCR产物进行SSCP分析,GLRaV-3 HSP70基因的扩增产物图谱也表现为2种带型(图 5a),结合在图谱中迁移的长度进行树状聚类分析(图 5b),发现13个分离物之间有明显差异,可归为2类:GLRaV-3 HSP70基因分离物LL-C,ZH-C,XN-S,DDT-S,XHB-H,GX-P,XHB-P,GX-XL和GX-M的相似性较高,带数为1条,迁移长度为2.3 cm,归为Ⅲa类;分离物DDT-C,XHB-S,LL-X和NDT-M的相似性较高,带数为2条,迁移长度为1.8 cm,归为Ⅲb类.分离物XHB-H,LL-X和NDT-M的聚类结果与GLRaV-3 CP基因的聚类结果不一致,可能存在碱基变异.
-
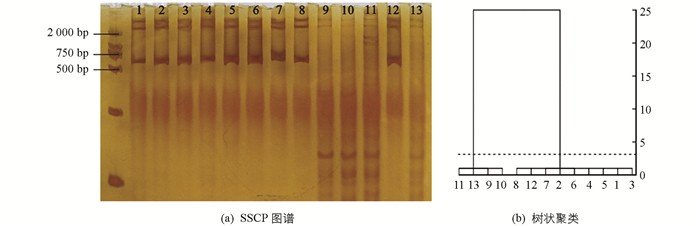
对13个GLRaV-3 RdRp基因阳性样本PCR产物进行SSCP分析,而GLRaV-3 RdRp基因的扩增产物图谱表现为3种带型(图 6a),结合在图谱中迁移的长度进行树状聚类分析(图 6b),发现13个分离物之间有明显差异,可归为3类:GLRaV-3 RdRp基因分离物ZH-C,XN-S,DDT-S,LL-X,GX-P,XHB-P,GX-XL和GX-M的相似性较高,带数为2条,迁移长度为2.5 cm,归为Ⅳa类;分离物LL-C,DDT-C和NDT-M的相似性较高,带数为3条,迁移长度为1.5 cm,归为Ⅳb类;分离物XHB-S和XHB-H的相似性较高,带数为2条,迁移长度为0.9 cm,归为Ⅳc类.
-
由表 3可知,应用PT-PCR和SSCP技术分析GLRaVs的遗传变异,对不同区域、不同品种分离得到的GLRaV-1和GLRaV-3种群遗传变异比较发现,同一地区不同品种、同一品种不同地区分离物检出GLRaV-1和GLRaV-3结果差异较大.阳性分离物DDT-C,XHB-S,DDT-S,XHB-P,XHB-H和LL-X均存在GLRaV-1和GLRaV-3的复合侵染现象,聚类分析结果也不相同,相互之间变异较大;永宁地区新惠彬酒庄葡萄园5大酿酒葡萄品种均检出GLRaVs,但检出结果不同;广夏三基地葡萄园3大酿酒葡萄品种均只检出GLRaV-3.从宁夏地区获得的13个GLRaV-3分离物之间存在着明显的地域分布性差异,且不同品种间也存在差异.永宁地区酿酒葡萄品种蛇龙珠GLRaV-3 CP,HSP70和RdRp基因的阳性分离物XHB-S和DDT-S分别归为Ⅱb,Ⅲb,Ⅳc类和Ⅱa,Ⅲa,Ⅳa类,且DDT-S与银川新牛酒庄葡萄园阳性分离物XN-S的GLRaV-3检测结果一致,但XN-S未检测到GLRaV-1.永宁地区酿酒葡萄品种霞多丽的阳性分离物LL-X和品丽珠的阳性分离物XHB-P将GLRaV-3归为Ⅱa,Ⅲa,Ⅳa类,同一地区的不同品种间GLRaV-3变异程度具有相似性.酿酒品种赤霞珠阳性分离物LL-C和ZH-C只检出GLRaV-3,XHB-C只检出GLRaV-1,而DDT-C存在复合侵染;检出GLRaV-1的聚类分析结果一致,但检出GLRaV-3的阳性分离物LL-C,ZH-C和DDT-C聚类分析结果差异较大,同一品种不同地区GLRaV-1和GLRaV-3的侵染结果不同,且变异较大.
2.1. DNA提取与质量检测
2.2. GLRaV-1 CP基因的RT-PCR检测
2.3. GLRaV-3 CP,HSP70和RdRp基因的RT-PCR检测
2.4. GLRaV-1和GLRaV-3 PCR产物的SSCP分析
2.4.1. GLRaV-1 CP基因PCR-SSCP分析
2.4.2. GLRaV-3 CP基因PCR-SSCP分析
2.4.3. GLRaV-3 HSP70基因PCR产物的SSCP分析
2.4.4. GLRaV-3 RdRp基因PCR-SSCP分析
2.5. 宁夏地区不同品种间GLRaV-1和GLRaV-3种群差异
-
宁夏贺兰山东麓酿酒葡萄产业发展早期,在葡萄病毒检测体系极不完善的情况下从美国、法国、河北昌黎和山东烟台等地引种栽培,在这种粗放式管理和大面积推广种植下,葡萄病毒得以快速在产区传播蔓延[23-24]. 1999年,宁夏玉泉营地区葡萄圃中26%的品种表现卷叶病症状,酿酒葡萄品种田间发病率为31.37%[25];2006-2009年,沙月霞等[10]的调查结果显示葡萄卷叶病发病率为36.0%;2016年,宁夏产区树龄8年以上的果园中主栽酿酒葡萄品种“蛇龙珠”和“黑比诺”田间自然发生葡萄卷叶病的发病率高达85.1%和52.7%[7].近年来,宁夏开展酿酒葡萄卷叶伴随病毒检测和无病毒苗木的培育工作时,主要检测出的3种病毒类型中GLRaV-1和GLRaV-3的检出率最高,GLRaV-3的研究最为广泛,同时存在GLRaVs的复合侵染现象[7, 9-10].葡萄卷叶伴随病毒病,属于RNA病害,由于RNA病毒复制快且缺乏校正机制,碱基错配重组极易发生[26],表现出高度变异. 2002年,刘命华等[27]对GLRaV-1 CP基因序列进行分析,发现其核苷酸和氨基酸同源性相对较低,暗示GLRaV-1分离物可能具有较高的遗传变异. 2011年,Alabi等[28]对美国34个GLRaV-1分离物的4个基因(CP,HSP70,CPd2和P24)进行多样性分析,发现GLRaV-1 CP基因的变异性较高.本研究结果表明,8个GLRaV-1 CP基因分离物有3种类型,表现出较高的变异性,这与上述研究结果基本一致.一些学者对获得的GLRaV-3分离物CP基因进行序列比对分析和多样性分析,结果显示CP基因是GLRaV-3基因重组较活跃的区域,可能存在着基因变异,不同分离物间的基因重组形成了新的变异种[29-30]. Turturo等[13]利用SSCP技术对45个葡萄样品GLRaV-3 CP,HSP70和RdRp基因的变异研究发现,上述基因存在多种不同类型的变异型,且GLRaV-3 CP基因的变异几率较大,而HSP70基因的变异几率较小,RdRp基因若发生变异定会引起GLRaV-3分子结构的变化.本研究结果显示,13个GLRaV-3 CP,HSP70和RdRp基因分离物分别有多种不同类型,与上述研究结果相类似,可能由于宁夏地区早期大面积推广苗木自繁、嫁接等措施导致病毒的复合侵染,增加了基因重组的几率,致使出现多种变异类型.但宁夏GLRaV-3分离物表现的不同类型是否由基因重组导致,还需要进一步检测.
-
利用RT-PCR技术对宁夏酿酒葡萄不同种植区的7个主栽品种的GLRaV-1和GLRaV-3进行分子检测,试验结果共获得8个GLRaV-1和13个GLRaV-3的阳性样本.说明GLRaV-1和GLRaV-3在宁夏不同种植区的酿酒葡萄品种间都有不同程度的侵染,且存在复合侵染.利用SSCP技术对扩增片段进行遗传变异分析,表明各分离物之间存在着明显的遗传变异. 8个GLRaV-1分离物基于CP基因分析差异明显,可分为3类;13个GLRaV-3分离物基于RdRp基因分析差异明显,可分为3类,而基于CP和HSP70基因分析可分为2类.说明宁夏酿酒葡萄不同种植区、不同品种的GLRaV-1和GLRaV-3种群分子特性存在差异且遗传变异明显.分离物的遗传变异不仅表现在种植区的分布上,同时也表现在不同品种间.



 下载:
下载: