-
核酸传递是生物学研究、转基因和基因治疗必经的重要关键环节.由于核酸酶的广泛存在和核酸的高分子量及生理生化特性,导致外源核酸很难独立地被递送进靶细胞,而少量递送入胞质的核酸又易被溶酶体-内涵体系统降解[1].因此,开发利用核酸运载体克服核酸转染屏障是生物学研究的重要问题.目前核酸运载体主要分为病毒类和非病毒类,尽管前者的转染效率高,但后者在安全性、稳定性、成本上要优于前者[2-3].
聚乙烯亚胺(Polyethylenimine,PEI)是一种水溶性阳离子高分子聚合物,化学结构中大量胺基正电氮原子使其携带高密度正电荷,早在1995年被用于体外核酸转染[4-5].带正电荷的PEI利用静电交互作用缩聚带负电的核酸并形成易被细胞摄取的正电小粒径复合体,这种凝聚核酸的能力极大地影响了转染效率[6]. PEI所含的大量胺基具有质子海绵效应,能促使PEI-核酸复合物逃离出内涵体和溶酶体继而在胞内释放核酸使目的基因得以表达,同时PEI可与细胞膜结构上的蛋白或磷脂相互作用影响膜的完整性,产生细胞毒性并影响细胞的转染效率[7-9].转染效率的提高及细胞毒性的控制是PEI介导核酸转染应用的关键. PEI的转染效率和细胞毒性受到PEI中的N元素与质粒DNA中P元素的摩尔数比(N/P)、PEI和核酸聚合及转染条件、PEI的分子量和分枝化等多种因素的影响[10-11].低分子量的PEI不能有效压缩聚合核酸,导致复合物表面电荷低,难以被细胞摄取[12].尽管PEI分子量的升高转染效率会升高,但毒性也会增强[13].大量研究最终确认25 kDa PEI是阳离子转染领域中的“黄金标准”[14-15]. PEI含分支化PEI (Branched PEI,BPEI)和线性PEI(Linear PEI,LPEI)两大类[16-17].研究表明BPEI虽然有着相对LPEI更强的核酸凝聚能力,但核酸释放能力仍很弱,进入胞内无法再有效释放核酸,同时其毒性也更大[16]. 25 kDa LPEI是一种相对于传统PEI毒性更小、转染效率更高的核酸阳离子载体[17]. N/P是影响PEI转染效率的最主要因素,但25 kDa LPEI N/P筛选研究差异较大,筛选的N/P范围(1~20)也较小[18-19]. PEI的转染效率也与LPEI-DNA复合体聚合程度和转染条件密切相关,研究表明不同PEI溶剂、盐离子浓度、冻融、血清、细胞密度等均会影响PEI的转运核酸能力[20-23]. LPEI作为核酸传递载体具有生物安全性高、操作简便、安全廉价等优点,具有作为基因治疗中核酸传递工具的应用潜力.但25 kDa LPEI介导核酸转染的条件缺乏系统性研究,其介导核酸转染的条件需要进一步筛选优化,以提高其携带核酸进行细胞内传递的效率.因此,本研究在前人研究的基础上,以适用性和认可度更好的25 kDa LPEI作为试验材料,探究N/P比,LPEI与DNA聚合时间,聚合pH对LPEI-DNA复合体聚合的影响,优化其对细胞转染的能力,为LPEI转染条件的完善及利用提供理论基础.
HTML
-
HEK293 T细胞由中国科学院干细胞库提供;LPEI(25 kDa),Santa Cruz公司;MTT,Sigma公司;DMEM和F12细胞培养基,Opti-MEM,胎牛血清,Gibco公司;胰酶细胞消化液,Beyotime公司;100x青霉素-链霉素溶液,Biosharp公司;绿色荧光基因质粒pEGFP由笔者所在实验室在pCDNA3.1载体中插入EGFP基因构建而成;DMSO,Sigma公司;HBS缓冲液(20 mmol/L),Hepes;150 mmol/L NaCl(pH值为7.1),其余试剂均为国产分析纯试剂.
-
聚合N/P比优化:按照核苷酸中平均330 Da含1个P,LPEI中平均43 Da含1个N,以此计算不同N/P所需的LPEI和核酸量[24].用HBS缓冲液配制特定N/P(0,1,2,3,4,6,8,10,20)的LPEI,经0.22 μm滤膜过滤后与质粒混合,配制总体积为20 μL的LPEI/DNA混合液,每份含1 μg质粒pEGFP.上述混合液在室温下静置30 min后,各取5 μL在1%琼脂糖凝胶上进行电泳,电泳条件为1×TAE电泳缓冲液,电压5 V/cm,电泳10 min用凝胶成像仪观察DNA条带的迁移并拍照.
-
用HBS缓冲液配制特定N/P(0,1,2,3,4,6,8,10,12,16,20)的LPEI与质粒混合液,分别静置10 min,30 min,1 h,2 h后按照上述相同电泳条件进行凝胶电泳阻滞试验.
-
利用1 mol/mL的NaOH溶液与酸度计精确调整HBS缓冲液和LPEI溶液的pH,按pH值为6,6.5,7,7.5,8分为5个组,每组分别配置N/P为2,4,6的LPEI与质粒混合液,按照上述相同电泳条件进行凝胶电泳阻滞试验.
-
HEK293 T培养在含有双抗(青霉素100 U/mL和链霉素100 μg/mL)和10%胎牛血清(FBS)的DMEM/F12培养液中.细胞培养条件:37 ℃,5% CO2,饱和湿度条件下,每2 d换液1次,待细胞生长达90%汇合状态时用胰酶消化法传代细胞.将细胞密度调整至2×105个/mL,以每孔400 μL细胞悬液接种24孔培养板,培养24~36 h,细胞汇合度达到80%左右时用于转染试验.
-
按照N/P比(0,1,2,6,10,20,30,40,60,80,100)每个处理5个平行孔.转染前细胞培养液换为400 μL Opti-MEM培养基,将且在pH值为6聚合1 h后的无菌25 kDa LPEI和pEGFP质粒(每孔的质粒量为1 μg)混合物滴加到对应的细胞孔中,轻轻晃动使转染物分布均匀.细胞继续培养4 h后换加Opti-MEM新鲜培养基,48 h后在荧光倒置显微镜下观察荧光细胞比例并拍照.利用Image-J软件进行绿色荧光阳性细胞计数分析,计算出转染效率.
-
将HEK293 T细胞按照转染效率检测试验的操作和分组均等接种于96孔板中,培养24 h后按照不同N/P比进行LPEI转染,每孔200 ng质粒(方法同1.6),每个处理5个平行.转染48 h后除去培养液,在每孔中加入20 μL MTT溶液(5 mg/mL)和80 μL新鲜的DMEM培养基,放入37 ℃培养箱培养4 h,移除MTT溶液,加入150 μL DMSO溶解紫色甲瓒结晶,用酶标仪在570 nm波长下测定每个孔的吸光度,计算细胞存活率:
-
数据统计使用SPSS 19,方差齐性通过Levene方法检测,并进行单因素方差分析(one-way ANOVA),Tukey's检验进行事后多重检验. p<0.05表示差异有统计学意义,数据表示方法均为平均值±标准差.
1.1. 细胞与试剂
1.2. 核酸阻碍试验
1.3. 聚合时间优化
1.4. 聚合pH优化
1.5. HEK293 T细胞培养
1.6. 转染效率检测
1.7. MTT检测
1.8. 统计学分析
-
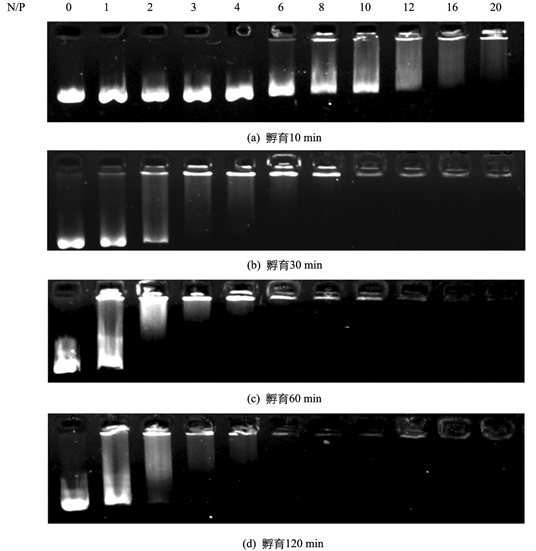
LPEI和DNA通过静电相互作用结合形成LPEI-DNA复合物,最终复合物正电性越高表明LPEI对DNA的凝聚效果越好,越容易被细胞摄取[1, 6]. LPEI-DNA复合物所带电荷的差异可以通过电场迁移的差异加以体现,复合物带正电荷增加会抑制或阻止质粒DNA在电场中的迁移.本研究首先利用凝胶阻滞试验分析了不同N/P对25 kDa LPEI与质粒DNA复合物形成的影响.结果显示,随着N/P升高,质粒DNA在琼脂糖凝胶电场中迁移能力逐渐受限且电泳条带变弱;当N/P在1~6时,部分质粒DNA在凝胶电场中迁移形成拖尾式亮带,当N/P超过6以后,质粒DNA甚至被完整滞留在电泳胶孔中(图 1).表明25 kDa LPEI相对于质粒DNA的量越多,起到的DNA凝聚效果越好.
-
本研究在不同N/P条件下利用琼脂糖凝胶电泳分析了LPEI和质粒DNA共孵育时间对LPEI-DNA复合物形成的影响.结果显示,当LPEI与质粒DNA孵育10 min进行电泳分析发现,N/P小于4时均未发现明显的核酸阻滞现象.当N/P在4~10时仅有微弱核酸阻滞现象,表明质粒DNA没有充分与LPEI结合(图 2a);当LPEI与质粒DNA孵育时间延长至30 min时LPEI与质粒DNA结合更加充分,N/P大于8时DNA在电场中的迁移完全被阻滞(图 2b);当LPEI与质粒DNA孵育时间延长至60 min,LPEI-DNA复合物聚合率达到最大,而孵育至120 min时相较于60 min泳道出现更严重的拖尾现象(图 2c,d).以上数据表明,LPEI与质粒DNA共孵育时间会显著影响LPEI-DNA复合物的形成及稳定性,在共孵育60 min前随着共孵育时间延长LPEI-DNA复合物稳定性增强,且能达到LPEI-DNA最高的结合效果;共孵育时间30~60 min时,最有利于LPEI-DNA复合物的形成,进一步延长聚合时间反而使LPEI-DNA复合物稳定性减弱.
-
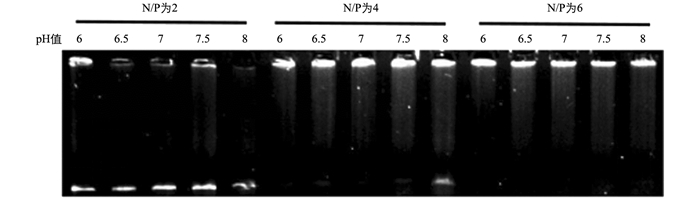
由于LPEI-DNA复合物是通过电荷互作形成的,因此LPEI与DNA共孵育的溶液pH可能是影响LPEI-DNA复合物形成的重要影响因素.本研究通过琼脂糖凝胶电泳阻滞试验分析了不同pH条件下LPEI与质粒DNA聚合规律.结果显示,当N/P为2时,随着pH的升高DNA在胶孔中滞留减少(图 3);当N/P为4和6时,不同pH条件下的LPEI-DNA复合物大部分滞留在胶孔中,但同样随着pH上升泳道核酸逃逸增多(图 3).以上结果表明,随着pH的升高LPEI聚合DNA的能力逐渐下降,而且随着N/P升高pH对LPEI-DNA复合物形成的影响逐渐减弱.
-
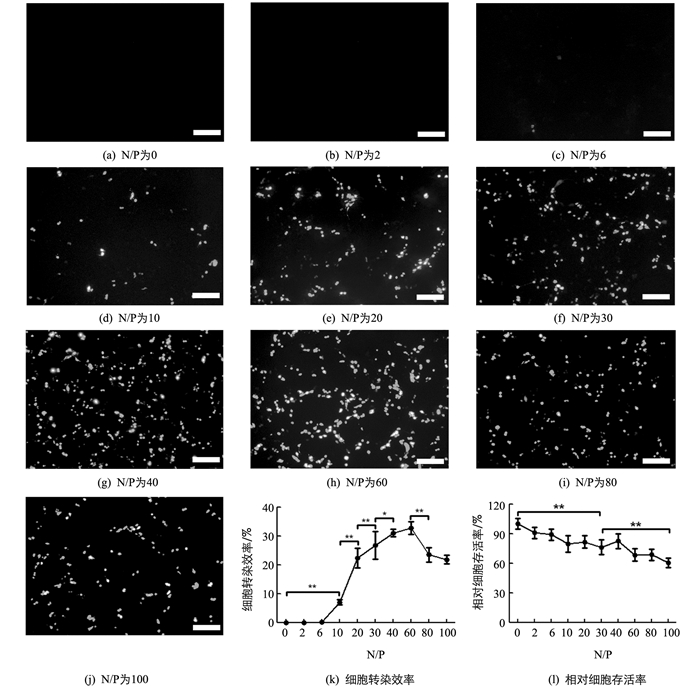
为了优化LPEI介导的细胞内DNA传递,本研究结合已有的筛选结果,确定LPEI和质粒DNA孵育时间为1 h,孵育的pH值为6,进一步筛选N/P对LPEI介导的DNA转染效率及细胞毒性的影响.本研究将不同N/P的LPEI-绿色荧光基因质粒(pEGFP)对HEK293 T细胞进行转染,48 h后通过在荧光显微镜下观察转染阳性细胞率,即绿色荧光蛋白表达情况,同时利用MTT法分析了LPEI对细胞活性的影响.结果显示,N/P<6时几乎没有绿色荧光信号(图 4a至图 4c);N/P超过10后绿色荧光细胞数量极显著增加(p<0.01),并随着N/P升高绿色荧光细胞数量逐渐增多,转染效率在N/P为60达到峰值,随后有所下降(图 4d至图 4k);LPEI对细胞活力影响的分析结果显示,随着N/P的升高细胞相对活力呈逐渐下降趋势(图 4l).当N/P达到30时,细胞活力相对于对照组极显著下降(p<0.01),当N/P达到60时,细胞活力进一步极显著下降(p<0.01),并能观察到被转染细胞出现大量悬浮和死亡的现象.
以上结果表明,随着N/P的上升转染效率呈先升高后降低的趋势,当N/P很低时(N/P为0~6),LPEI无法有效向细胞内传递核酸;当N/P为40~60时,最有利于25 kDa LPEI向细胞传递核酸,而进一步升高N/P会造成游离LPEI增加,产生细胞毒性作用,造成大量细胞死亡.
2.1. N/P对LPEI-DNA复合物形成的影响
2.2. 聚合时间对LPEI-DNA复合物形成的影响
2.3. pH对LPEI-DNA复合物形成的影响
2.4. N/P对LPEI介导的DNA转染效率的影响
-
25 kDa LPEI是一种有效的核酸和药物传递载体,制备方便,价格低廉,其核酸转染功能被广泛应用[25-26].为了更有效地利用25 kDa LPEI这一核酸转染工具,本研究通过凝胶阻滞试验研究N/P,LPEI与DNA聚合pH,聚合时间对25 kDa LPEI聚合DNA能力的影响,并通过细胞转染试验研究了大范围N/P对LPEI介导的DNA转染效率及细胞毒性的影响.
PEI聚合核酸的能力与其化学骨架中携带阳离子的胺基量密切相关.有研究表明,胺基相对于核酸的量,即N/P升高时,PEI与核酸形成的正电复合体表面电势变大,粒径变小,PEI对核酸的吸附和压缩能力增强[24].本研究中不同N/P的25 kDa LPEI-DNA复合物凝胶阻滞试验表明,随着N/P的增加,核酸在电泳中受到的阻滞作用越强.当N/P较高时,核酸在琼脂糖凝胶中的迁移被完全抑制.表明N/P的增加有助于25 kDa LPEI对核酸的聚合及阳离子化,这符合大多数PEI或修饰性LPEI传递核酸的规律[27-28].
本研究中不同聚合时间下25 kDa LPEI-DNA复合物凝胶阻滞试验表明,25 kDa LPEI与DNA共孵育10 min无法有效形成稳定的LPEI-DNA复合物,而在共孵育60 min时LPEI与DNA聚合达到饱和,120 min时出现核酸逃逸现象.表明25 kDa LPEI与核酸聚合反应时间低于10 min或超过1 h均不利于形成LPEI-DNA复合物,最适聚合时间为1 h.研究表明22 kDa LPEI与DNA共孵育时间过长,LPEI-DNA复合物粒径将迅速变大,并逐渐趋于不稳定[29-30].
PEI与核酸共孵育环境可以通过离子强度、渗透压来影响聚合物表面电势及粒径[31-32].本研究中不同聚合pH下25 kDa LPEI和DNA复合物凝胶阻滞试验表明,较低的pH有利于LPEI对核酸聚合,最适聚合pH值为6.有研究报道酸性条件可以增加PEI电势,但对细胞转染效率无显著影响[33].本文推测尽管pH的降低有利于LPEI对核酸的聚合,但聚合过程中LPEI的H+过度富集导致LPEI阳离子缓冲性减弱,最终可能影响LPEI-DNA复合物逃逸溶酶体,这一推测还需研究验证.
研究表明阳离子载体的质子缓冲能力越强其对细胞的核酸递送能力越强,而前人研究已表明LPEI的质子缓冲能力与N/P密切相关[1, 34].本研究中不同N/P对HEK293 T细胞的转染试验表明,N/P小于10时LPEI难以介导核酸转染,随N/P升高转染效率逐渐增强,在N/P为60时转染阳性细胞达到峰值,这与前人得出的PEI,BPEI,10 kDa LPEI的最佳转染N/P为6~10的结论有所不同,本文推测这可能与试验采用的LPEI分子以及聚合条件不同有关,同时前人试验中认为LPEI的毒性过强会影响转染效率导致N/P的筛选范围过小[28, 35-36].
一定浓度的游离LPEI可在细胞表面吸附和聚集引起膜结构的融合、改变,也可以渗透入核造成DNA损伤,最终导致细胞死亡,而DNA与LPEI聚合后反而会降低毒性,因此恰当的N/P选择对细胞活率尤为重要[34, 37].有研究表明,细胞毒性过大,进而造成细胞死亡降低转染效率[38].而本研究显示,随N/P增加细胞毒性增大,当N/P大于60后转染效率下降,但细胞活率变化不明显,表明转染效率的降低与细胞毒性的增加没有相关性.本研究还推测N/P过大造成转染效率降低的原因可能是过量LPEI过度结合DNA,导致DNA无法脱离LPEI-DNA复合体,从而DNA难以进入细胞核表达,这一推测还需研究验证.本研究表明,满足转染效率的同时并结合细胞活率筛选确定N/P为40~60可能是细胞转染的最佳N/P范围.
除此之外,科学家还通过对LPEI进行修饰、交联、螯合等不同方式提高转染效率或降低细胞毒性[39-41].随着LPEI优化研究的不断深入,多项研究表明该系统具有治疗肝癌、结肠癌、膀胱癌等重大疾病的重大潜力[42-44].本研究对25 kDa LPEI介导核酸转染的优化以及合适转染N/P范围的筛选能为后续生物学与基因治疗研究提供参考依据以及应用基础.







 DownLoad:
DownLoad: