-
马尾松(Pinus massoniana Lamb.)是我国南方地区造林先锋树种,具有适应性强、用途广、速生丰产等优点,贵州省马尾松资源分布不均衡、林地生产率低,且低效林面积日益增加[1-2].杜鹃(Rhododendron simsii Planch.)常伴生于马尾松林下,对维持马尾松林稳定性、提高林地生产力具有重要意义[3].植物与共生真菌之间的相互影响可以促进和维持两者地上和地下的多样性[4],充分了解土壤真菌的多样性和群落结构,对有效促进农林牧业的生产,维持生态系统物种的多样性、保持生态平衡等有重要意义[5].菌根围作为土壤-根系-微生物三者紧密结合且相互影响的区域[6],承载着植物、土壤和微生物之间的绝大部分物质、能量与信息的交换[7].真菌作为植物根围土壤中微生物的重要组成部分,既能调节微生物数量和种群结构,抑制病原菌生长[8-10],还能影响植物根系对矿质营养的吸收和分配,增强植物的抗逆性[11-14].
目前已有大量有关不同生境下的马尾松林外生菌根(Ectomycorrhiza,ECM)[15]和杜鹃属植物菌根(Ericoid Mycorrhiza,ERM)多样性的研究报道[16-19],Heinonsalo等[20]对同一生境下的杜鹃属菌根及伴生植物菌根进行比较研究,得出杜鹃属菌根真菌在其他植物根部也能形成,共生植物菌根之间存在关联性.黔中地区马尾松纯林较广,杜鹃作为伴生植物增加了森林物种的多样性,但有关马尾松-杜鹃群落土壤真菌多样性的研究还罕见报道.笔者以杜鹃根围土壤作为研究对象,探讨马尾松-杜鹃群落杜鹃根围土壤真菌的多样性,在黔中地区选取3个相似植被类型的马尾松-杜鹃混交林样地,以扩增子(internal transcribed spacer,ITS)区作为测序研究区,通过数量生态学原理分析植物多样性及土壤养分对真菌群落的影响,为后续马尾松纯林改造、马尾松-杜鹃复合林地维护及森林组成、结构和多样性的调整奠定基础.
HTML
-
试验样地选取黔中地区的贵阳市乌当区百宜乡(WD)、花溪区孟关(MG)和贵州省黔南龙里县龙架山森林公园(LL)3个地区为样地,研究区植物群落从上到下依次为乔木层、灌木层、草本层,其中马尾松-杜鹃为样地常见植物群落.马尾松树高15~20 m,胸径25~35 cm;杜鹃高1.75~2.40 m,地径2.5~3.2 cm.此外,样地伴生植物还有白栎(Quercus fabri Hance)、茅栗(Castanea seguinii Dode)等多种灌木.黔中地区属亚热带季风性湿润气候,土壤以山地黄壤为主,呈酸性,土质黏重.年均温约15.3 ℃,年降雨量高于870 mm,平均相对空气湿度79.9%,日照百分率25.7%,无霜期200 d以上.
-
于2017年5月15日、17日、20日分别在3个样地选取马尾松-杜鹃生长良好且杜鹃长势相似的群落,建立20 m×20 m的标准样方[21],去除杜鹃垂直盖度以下的枯枝落叶层和杂物,由主干追踪到侧根和发根区域,挖出5~20 cm的杜鹃根段主要分布层,剪取粗壮发根并用毛刷刷取附着其上0~1 mm的根围0.5 g土壤用作杜鹃根围真菌多样性分析[22].每个地区选择3个样方采集3个样品,每个样品之间间隔超过5 m,试验一共9个样品,同时采取根际层(5~20 cm)用作土壤养分测定.试验工具在采样之前均经过严格灭菌处理,样本采集后立刻用液氮处理,带回后保存在-20 ℃冰箱中备用.
-
详细调查样方中的所有植物种类与数量,对样地植物进行α多样性分析:采用Shannon-Wiener多样性指数(D)和Pielou均匀度指数(J)分析3个样地植物多样性特征.
式中S为物种数目,N为样地所有植物总数之和,Pi为第i种植物所占的比例,Pi=Ni/N,Ni为i种植物数量.根据鲍士旦《土壤农化分析》测定土壤pH值和有机质、全氮、速效氮、速效磷和速效钾(表 1).
-
采用Illumina MiSeq二代测序平台对样本DNA片段进行双端测序(由上海派森诺生物测序公司完成).利用试剂盒omega EZNA soil extration kit提取杜鹃须根根围土壤中的真菌DNA样本,利用0.8%的凝胶电泳和紫外分光光度计检测DNA的质量和浓度.检测合格后,利用微生物rDNA-ITS保守区域设计引物,上游引物:5′-GGAAGTAAAAGTCGTAACAAGG-3′;下游引物:5′-GCTGCGTTCTTCATCGATGC -3′[23].进行特异性扩增PCR反应程序为:98 ℃预热变性2 min;25个循环,每个循环98 ℃,热变性15 s,55 ℃退火30 s,72 ℃延伸30 s;最后72 ℃延伸5 min,10 ℃保存.将扩增产物进行回收、荧光定量,文库制备合格后上机测序.测序得到的基因序列提交至GenBank数据库https://www.ncbi.nlm.nih.gov/sra/SRP133922,登录号:SRP133922.获得原始数据后,对测序质量进行评估和质控,利用QIIME软件将OTU (Operational Taxonomic Units)归并和分类(相似度阈值为97%)[24],采用UNITE数据库[25]进行OTU划分并对分类地位进行鉴定.获得OTU丰度矩阵之后,计算每个样本群落的Alpha多样性[26-29].
采用SPSS 2.0软件,运用差异显著法,分析样地植物α多样性、土壤养分、杜鹃根围土壤真菌α多样性.利用Python 2.7软件对组间显著性差异菌群作Linear discriminant analysis effect size (LEfSe)分析.
运用数量生态学原理,利用R语言ape包、vegan包、gbmplus包对真菌纲水平作主坐标分析(Principal Coordinates Analysis,PCoA),对菌群结构与生境因子(植物α多样性、土壤养分)之间作冗余分析(Redundancy Analysis,RDA).
1.1. 研究地概况
1.2. 采样方法
1.3. 样地植物调查和土壤养分的测定
1.4. 菌根围真菌DNA提取及多样性分析
-
试验共获得有效序列505 444条,count值最多的是265 bp,达44 656条;主要区间范围为190~290 bp,length值主要为2 000~20 000.总共检测到3 435个OTU,鉴定结果分属于9门23纲84目169科389属真菌,其中LL杜鹃根围检测到真菌9门19纲53目90科127属(495个OTU),WD的真菌有8门21纲78目150科246属(1 076个OTU),MG则有6门17纲55目99科129属(522个OTU).所测得的OTU数量从大到小依次为WD,MG,LL,共有OTU数量162个,WD特有的OTU数量为685个,MG和LL分别为192个和158个,3个样地根围真菌OTU种类和数量差异较大.
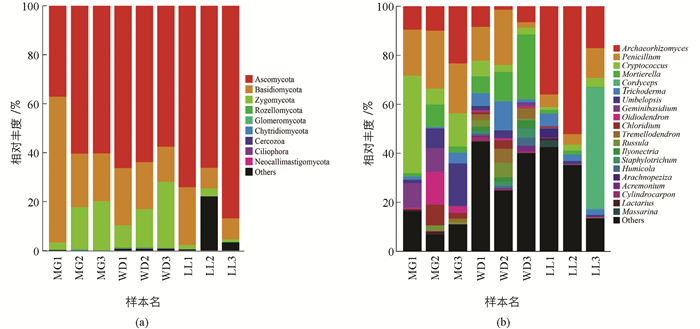
根据菌群OTU阀值归并得出所有OTU主要分属于9门(Plylum)真菌:Ascomycota(63.6%),Basidiomycota(22.0%),Zygomycota(10.8%),Rozellomycota(0.2%),Glomeromycota(0.1%),Chytridiomycota(0.0%),Cercozoa(0.0%),Ciliophora(0.0%),Neocallimastigomycota(0.0%) (图 1a). Ascomycota占比最高,在WD,MG,LL所占比例分别是62.58%,52.63%,75.71%;其次是Basidiomycota,所占比例分别是WD(33.62%),MG(18.97%),LL(13.515%).
9个菌门分类鉴定得到23纲,鉴别得丰度较高的10个纲依次为:Sordariomycetes(18.9%),Archaeorhizomycetes(18.3%),Eurotiomycetes(15.1%),Agaricomycetes(10.8%),Tremellomycetes(9.5%),Leotiomycetes(4.4%),Dothideomycetes(2.4%),Wallemiomycetes (2.4%),Incertae sedis (1.0%),Pezizomycetes (0.6%).其中优势纲Sordariomycetes占比分别为MG(7.96%),WD(24.88%),LL(23.96%);其次是Archaeorhizomycetes,占比分别为MG (14.28%),WD (5.44%),LL(35.08%).
84目中丰度较高的10个目:Archaeorhizomycetales(18.3%),Eurotiales (14.1%),Hypocreales(13.4%),Tremellales(9.1%),Mortierellales(6.8%),Sebacinales (4.6%),Mucorales (3.8%),Geminibasidiales (2.4%);Incertae sedis(2.4%),Chaetosphaeriales(2.0%).优势菌目Archaeorhizomycetales占比分别为MG (14.28%),WD (5.44%),LL (35.08%);其次是Eurotiales,占比分别为MG(21.40%),WD(13.55%),LL(7.31%).
169科中丰度较高的10个科依次为:Archaeorhizomycetaceae(18.3%),Trichocomaceae (14.1%),Mortierellaceae(9.1%),Cordycipitaceae(6.8%),Hypocreaceae(5.9%),Umbelopsidaceae(3.7%),Sebacinales.Group.B (2.9%),Geminibasidiaceae (2.4%),Myxotrichaceae (2.2%),Chaetosphaeriaceae(2.0%).优势科Archaeorhizomycetaceae占比分别为MG(14.28%),WD(5.44%),LL(35.08%);其次是Trichocomaceae,占比分别为MG(21.40%),WD(13.51%),LL(7.31%).
389属真菌中优势菌群有:Archaeorhizomyces(18.3%),Penicillium(13.7%),Oidiodendron(2.1%),Cordyceps(5.5%),Trichoderma(3.9%),Chloridium(1.8%),Cryptococcus(8.8%),Geminibasidium(2.4%),Tremellodendron(1.5%),Russula(1.4%),Mortierella(6.8%),Umbelopsis(3.7%)等20个属(图 1b).其中LL地区前3个优势属为Archaeorhizomyces(35.08%),Cordyceps(16.58%),Penicillium(7.20%);WD地区为Mortierella(15.09%),Penicillium(12.85%),Trichoderma(6.10%);MG为Penicillium(20.95%),Cryptococcus(19.89%),Archaeorhizomyces(14.28%).此外,在能识别到的真菌属中,MG特有真菌有Letrouitia,Inocybe,Brachyphoris,Placopsis,Suillus等,比例为36.3%;WD特有真菌有Harzia,Chalara,Verticillium,Odontia,Bandoniozyma等,占6.9%;LL特有真菌有Wilcoxina,Hydnocystis,Sympodiella,Chaenothecopsis,Boletinellus等,占5.6%.
菌群多样性分析如表 2所示,3个地区菌群Simpson指数差异不显著;Chao1指数和ACE指数结果相似,WD显著大于MG和LL,而LL和MG之间没有显著差异;Shannon指数也是WD显著大于MG和LL,MG与LL之间差异不是很大.
-
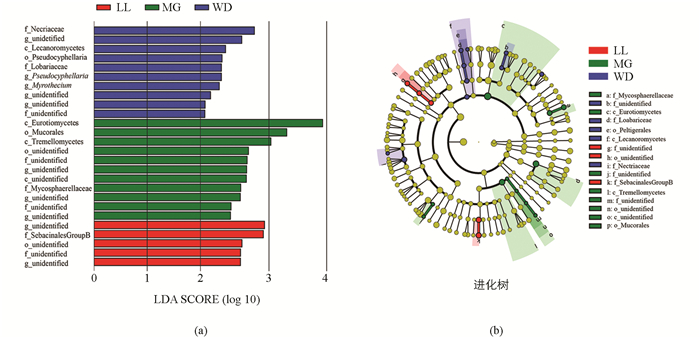
取菌群169科相对丰度最大的前100科,运用LEfSe分析组间显著性差异菌群,其中分值大于3.0的真菌种类有34个,结果见图 2a.组间差异分类单元如图 2b所示,LL差异指示菌为Archaeorhizomycetales和Archaeorhizomycetaceae;MG为Orbiliales和Orbiliaceae;WD地区差异指示菌有:Geoglossales Geoglossaceae,Incertae sedis,Peltigerales Collemataceae;Orbiliales Orbiliaceae,Leptosphaeriaceae;Hypocreales Bionectriaceae,Incertae sedis,Nectriaceae;Incertae sedis,Melanosporales,Ceratostomataceae;Xylariales Xylariaceae,unidentified;Boletales;Diplocystidiaceae,Boletaceae;Clavulinaceae;Clavulinaceae;Ganodermataceae,Polyporales;Sebacinaceae.可以看出,乌当地区菌群差异指示菌最多,达到9个目18个科,而LL,MG分别只有1个目和1个科.
-
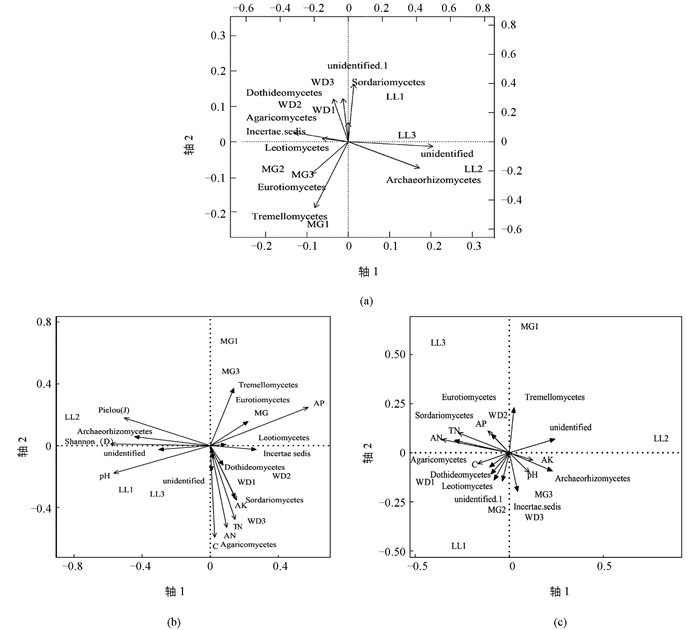
为了更好地了解杜鹃根围真菌分布的规律,对10个优势菌纲做主坐标(PCoA)分析(图 3a).得出LL地区主要的优势菌纲是Sordariomycetes,Archaeorhizomycetes和2个不确定的纲;WD地区主要的优势菌纲有Sordariomycetes,Leotiomycetes,Dothideomycetes和Incertae sedis;MG地区的优势菌纲为remellomycetes和Eurotiomycetes. RDA分析植物群落物种多样性对前10个优势菌纲分布的影响(图 3b),第1和第2主轴的累积方差贡献率为65.26%;其中第1主轴解释真菌纲水平组成方差变异的38.20%,第2主轴则为27.06%;Shannon多样性指数(D)、Pielou均匀度指数(J)与Archaeorhizomycetes呈正相关关系,但与其他9个纲的真菌呈负相关关系.利用偏冗余分析,以植物多样性为协变量、土壤养分为解释变量、真菌优势纲为响应变量,分析土壤养分对真菌群落结构的影响(图 3c).第1和第2主轴累积方差贡献率为66.78%,其中第1主轴解释真菌纲水平组成方差变异的38.26%,第2主轴则为28.52%.土壤养分对3个区系的真菌纲水平的影响从大到小依次为AN,TN,AP,C,pH值,AK,而且AN,TN与AP具有明显的协同作用;AP,AN,TN与Eurotiomycetes,Sordariomycetes,Tremellomycetes和unidentified菌群分布成正相关性,对其影响最大;而C与Agaricomycetes,Dothideomycetes,Leotiomycetes,unidentified.1呈负相关性;pH值,AK与Archaeorhizomycetes,Incertae sedis呈负相关性.由此得出真菌群落结构与生境土壤AN,TN,AP,AK,pH值,植物多样性、均匀度之间密切相关.再者,结合菌群生境环境因子分析(表 1),得出马尾松-杜鹃群落杜鹃菌根围土壤真菌群落结构主要受土壤pH值,TN,AN,AP,C及植物Shannon多样性的综合影响.
2.1. 不同区系杜鹃菌根围真菌多样性
2.2. 根围真菌科水平群落结构
2.3. 生境环境因子对真菌群落的影响
-
黔中地区马尾松-杜鹃群落杜鹃根围拥有丰富的真菌多样性,优势菌群主要为子囊菌门(Ascomycota)和担子菌门(Basidiomycota)真菌.子囊菌门有古生菌纲(Archaeorhizomycetes)、散囊菌纲(Eurotiomycetes)、锤舌菌纲(Leotiomycetes)等;担子菌门有银耳纲(Tremellomycetes)、节担菌纲(Wallemiomycetes)、伞菌纲(Agaricomycetes)等.已有研究表明,土壤真菌的多样性与地上部分植被的多样性和生态环境相关[30],菌根类植物经常都拥有相似的真菌共生体,真菌的类型取决于周围环境的植物类型[31-32].马尾松菌根为外生菌根(ECM),杜鹃菌根为杜鹃属植物菌根(ERM),研究发现两者共同生活的栖息地拥有丰富的菌根菌类型.杜鹃根围优势真菌有ECM伞菌纲的伞菌属(Agaricomycetes)和红菇属(Russula),也有ERM锤舌菌纲树粉孢属(Oidiodendron)等.根围作为杜鹃与外界信息交流的重要枢纽,推测周围生长的马尾松菌根是外生菌根菌的重要来源.此外,研究发现的优势真菌已有大量报道,Rosling等[33]研究古生菌(Archaeorhizomyces)发现其普遍存在于松属、杜鹃属和其他阔叶树的根和根际土壤中,表现出生态系统和寄主栖息地特异性,其形成的菌根菌包括数百种复杂且不可识别的丝状菌根结构,并具有腐生潜力.被孢霉属(Mortierella)、木霉属(Trichoderma)和青霉属(Penicillium)具有腐生能力,能够分解华山松针叶凋落物,对生态系统养分循环具有促进作用[34-35].青霉属、红菇属、蜡壳耳属(Sebacina)、隐球菌属(Cryptosporiopsis)等在不同林型马银花(Rhododendron ovatum)根部也有发现[36].可以看出,受植物类型的影响,马尾松-杜鹃群落杜鹃根围拥有丰富的真菌,潜在功能性极高,具有很大的真菌资源开发价值和生态研究意义.
-
LEfSe分析显示,黔中3个地区之间菌群结构差异较大.前100个科真菌差异指示种中,乌当(WD)地区菌群差异指示种达到9个目18个科,而龙里(LL)、孟关(MG)分别只有1个目和1个科.这种差异可能是由于树种特性和丰富度、土壤理化性质因素造成[37].从菌群Alpha多样性来看,Chao1,ACE,Shannon指数均为WD显著大于MG和LL,而LL和MG之间没有显著差异. WD地区土壤pH值显著高于MG和LL,而MG和LL差异不显著,据Priyadharsini等[10]报道,pH值影响真菌生长的环境并决定土壤中物质的存在状态,推测pH值为影响WD地区菌群多样性的重要原因之一.不同区系真菌OTU数量从大到小依次为WD,MG,LL,植物Shannon多样性指数(D)从大到小依次为WD,MG,LL,WD地区菌群OTU数量最大,植物多样性也最丰富.植物在空间和时间上的异质性投入增加了土壤生物的多样性[38],植物多样性对生态系统产生积极影响,如增加森林生物量和土壤有机质的储存,增加土壤微生物对根际光合碳的利用途径;同样,微生物的相互作用可以驱动生态系统的功能多样性,如植物多样性增加,生产力提高和应变能力增强等[39-40].
受植物种、植物群落和环境因子的相互作用,植物组成接近的生态群落,其环境因子组成上也比较接近,土壤环境和植物多样性的差异促进了菌群数量和结构的多样化[41]. RDA分析显示,真菌的群落结构受生境植物多样性和土壤养分综合影响,造成了其群落结构的多样性,其中真菌群落结构与土壤pH值,AN,TN,AP及植物的Shannon指数(D)相关性较大.与何苑皞等[42]、Dickie等[43]的结论类似,土壤真菌群落多样性指数与林下植被多样性、土壤全氮含量呈显著正相关关系.也有研究者提出森林土壤真菌多样性与植物样性呈负相关关系,森林密度非常高的情况下,容易形成单个物种的纯林模式,植物多样性减少,植物菌根菌类型也减少,但其他真菌类型增加[44].本研究得出WD地区马尾松-杜鹃混交林植物多样性最高,杜鹃根围真菌也最丰富,推测丰富的植物与土壤真菌形成了稳定的森林生态系统,真菌多样性与生境植物样性表现出一致规律.这与Kernaghan[4],Priyadharsini等[10]的观点相似,植物分泌富含碳的有机物和激素等物质影响根围土壤微生物的种类和数量,真菌促进植物对矿质元素和水分的获取,不同营养方式的菌根菌与其他非菌根菌获取碳源的方式影响其与宿主的相互选择关系.
此外,影响菌群结构的一个重要原因是真菌的扩散限制.外生菌根真菌具有长距离分散作用,而丛枝菌根真菌和其他类型菌根菌由于长距离扩散的效果较差,早期演替过程中表现出较小的可预测性和随机性[45].研究仅对土壤养分和植物群落物种α多样性进行分析,植物根系分泌物的成分及其他环境因子还不了解,种源差异、生长地的海拔差异都会影响杜鹃根际土壤微生物多样性及其群落结构[46],在今后的研究中将进行更深入的探讨和长期观察.
-
黔中地区马尾松-杜鹃群落杜鹃根围土壤真菌多样性丰富,不同地区菌群群落结构受植物群落物种α多样性、土壤养分的综合作用导致差异较大,植物多样性丰富的区系真菌多样性也最丰富.土壤pH值、速效磷、有机质、全氮、速效氮与菌群多样性之间存在显著相关性.






 DownLoad:
DownLoad: