-
吲哚美辛(Indomethacin,IMC)是一种非甾体抗炎药[1-3] (Nonsteroidal Antiinflammatory Drugs,NSAIDs),通过抑制环氧合酶使前列腺素的合成减少、抑制中性粒细胞迁移、白细胞的聚集和血小板的凝集等方式发挥抗炎作用[4-5],在临床上广泛用于急性风湿性及类风湿性关节炎、骨关节炎、强直性脊柱炎和多种发热症状的缓解.吲哚美辛属于BSC Ⅱ类药物,其在水中溶解度极小,为0.937 mg/L[6],油水分配系数为4.10,有低溶解性和高渗透性的特点. IMC经过口服给药后,它的释放或溶出较为缓慢,生物利用度较低.为了克服难溶性药物水溶性差的缺点,目前常常将这类药物制备成盐[7]、药物前体[8]、络合物[9]、胶束[10-11]、微乳[12-14]、纳米乳[15]、纳米混悬剂[16]、固体脂质纳米粒[17]以及固体分散体[18-20]等.
药物的固体分散体是一种应用较为广泛的药物制剂技术,它可以延缓或控制药物释放、增加难溶性药物的溶解度和溶出率、提高药物的化学稳定性.但固体分散体存在载药量小、长期储存后易老化等缺点.由于介孔二氧化硅特殊的物化性质,可以提高药物的载药率及溶出度、增加药物的稳定性等.因此,本文以吲哚美辛(IMC)为模型药物,以PVPk30和介孔SiO2为共同载体,制备IMC-PVPk30-SiO2固体分散体复合物,同时对其表征,测定载药率及溶出率,旨在为难溶性药物构建新的递药系统提供新思路.
HTML
-
UV-6100紫外分光光度计,上海美谱达仪器有限公司;DSC-200PC型差示扫描量热仪,德国NETZSCH公司;JSM 5400LV型扫描电镜,日本电子株式会社;RCZ-6B型溶出仪,上海黄海药检仪器厂.
-
吲哚美辛原料:质量分数99.0%,批号20170321,上海盛欣医药化工有限公司;吲哚美辛对照品:质量分数99.5%,批号20170226,上海盛欣医药化工有限公司;PVPk30:上海阿拉丁生化科技股份有限公司;介孔SiO2(Syloid SP53D):美国Grace公司;磷酸盐缓冲液(PBS 0.01 mol/L,粉剂):上海鼎国生物技术有限公司.
-
精密称取一定量的吲哚美辛和PVPk30(质量比为1:1),分别用适量的无水乙醇溶解后,合并两溶液,搅拌均匀.分多次将溶液转入茄形烧瓶中,用旋转蒸发仪除去溶剂(水浴温度为50 ℃,转速为100 r/min).将残余物取出,置于不锈钢板上,60 ℃真空干燥24 h.收集干燥后的产物,研细后过80目筛,即制得IMC-PVPk30固体分散体.
-
精密称取一定量的吲哚美辛和介孔SiO2(质量比为1:1),用适量的无水乙醇超声溶解吲哚美辛.将溶液转入西林瓶中,随后加入SiO2.用磁力搅拌器搅拌24 h后,分多次将瓶中溶液转入茄形烧瓶中.用旋转蒸发仪除去溶剂(水浴温度为50 ℃,转速为100 r/min).将残余物取出,置于不锈钢板上,60 ℃真空干燥24 h.收集样品,研细,过80目筛,即得IMC-SiO2固体分散体.
精密称取一定量的吲哚美辛、PVPk30和介孔SiO2(质量比为1:1:1),分别用适量的无水乙醇溶解吲哚美辛和PVPk30,合并两溶液,搅拌均匀.随后加入SiO2,后续操作同上述制备IMC-SiO2固体分散体步骤,制得IMC-PVPk30-SiO2固体分散体复合物.
-
分别称取制备的样品约5 mg,置于铝坩埚中,按以下条件进行测试.实验条件为,标准物:铟;热分析扫描速率:10 ℃/min;载气:氮气;温度范围:40~200 ℃.
-
将制备的样品粉末干撒在贴有导电胶带的样品座上,喷金处理5 min,取出,置于电镜测试仪器下观察,测试条件为15 kV.
-
利用已建立的紫外-可见分光光度法测定固体分散体及复合物中吲哚美辛的质量分数.精密称取制备的样品20.0 mg,置于50 mL的容量瓶中,用50%乙醇溶液溶解并定容,过滤,取滤液于320nm处测定吸光度,平行测定3次.利用回归方程计算吲哚美辛质量.样品中吲哚美辛质量分数(C吲哚美辛)计算公式如下[21]:
-
参照2015版《中国药典》的相关内容,选用磷酸盐缓冲液(pH值为7.2)与水的比例为1:4作为溶出介质之一.由于吲哚美辛在pH值为6.8缓冲液(人工肠液)中的溶解度较高,能够达到漏槽条件,且比较接近体内环境,故同时选择人工肠液为固体分散体和复合物溶出度测定的介质.
-
在2个溶出杯中分别加入750 mL的上述两种溶出介质,转速为75 r/min,温度为(37±0.5) ℃.精密称取相当于50 mg IMC的固体分散体及复合物样品,填装于0号胶囊中.将胶囊投入溶出介质中,分别于5,10,15,20,25,30,45,60,90,120 min时间点定时定位取样10.0 mL.经0.22 μm微孔滤膜过滤,弃去初滤液并即时补充相同温度、相同体积的溶出介质.立即取出样品在320 nm处测定吸光度值,依据标准曲线的回归方程,计算IMC质量分数,得到IMC在不同时间点的累积释放度,绘制时间-释放度曲线.
1.1. 材料
1.1.1. 仪器与设备
1.1.2. 药品与试剂
1.2. 实验方法
1.2.1. 载药二氧化硅固体分散体及复合物的制备
1.2.1.1. 溶剂蒸发法制备IMC-PVPk30固体分散体
1.2.1.2. 浸没-溶剂蒸发法制备IMC-SiO2固体分散体和IMC-PVPk30-SiO2固体分散体复合物
1.2.2. 载药二氧化硅固体分散体及复合物的表征
1.2.2.1. 差示扫描量热法(DSC)
1.2.2.2. 扫描电镜(SEM)
1.2.3. 固体分散体及复合物中吲哚美辛的载药率测定
1.2.4. 固体分散体及复合物中吲哚美辛的溶出度测定
1.2.4.1. 溶出介质
1.2.4.2. 测定方法
-
图 1为制备得到的固体分散体及复合物干燥24 h后的外观形态. 图 1a为IMC-PVPk30,呈浅黄色薄片状固体. 图 1b中,左侧为IMC-SiO2,样品干燥后呈白色粉末状固体;右侧为IMC-PVPk30-SiO2,样品干燥后呈黄色固体.
-
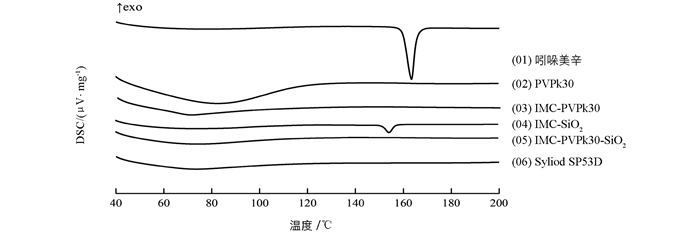
如图 2所示,吲哚美辛在163.6 ℃处具有一尖锐熔融峰,即为吲哚美辛晶体的熔点峰. PVPk30为非离子型高分子聚合物,故PVPk30无固定熔点,熔融峰在83.4 ℃附近.介孔SiO2在40~200 ℃范围内无熔融峰出现.
在IMC-PVPk30中,71.1 ℃处有一不明显的熔融峰,无IMC的熔点峰,说明成功制备了IMC-PVPk30,IMC在固体分散体中以无定形状态存在.在约154 ℃处,IMC-SiO2样品出现一较小的熔融峰,说明IMC与SiO2形成了低共熔物,IMC可能以微晶[22]或无定形状态分散于载体中.
在IMC-PVPk30-SiO2中,IMC熔融峰消失,说明固体分散体制备成功.相较于IMC-PVPk30和IMC-SiO2,IMC-PVPk30-SiO2中的IMC完全以无定形态存在. PVPk30,SiO2内(外)表面的羟基可与吲哚美辛的羧基形成氢键,有利于维持IMC-PVPk30-SiO2固体分散体复合物体系的稳定,延缓其老化的发生.
-
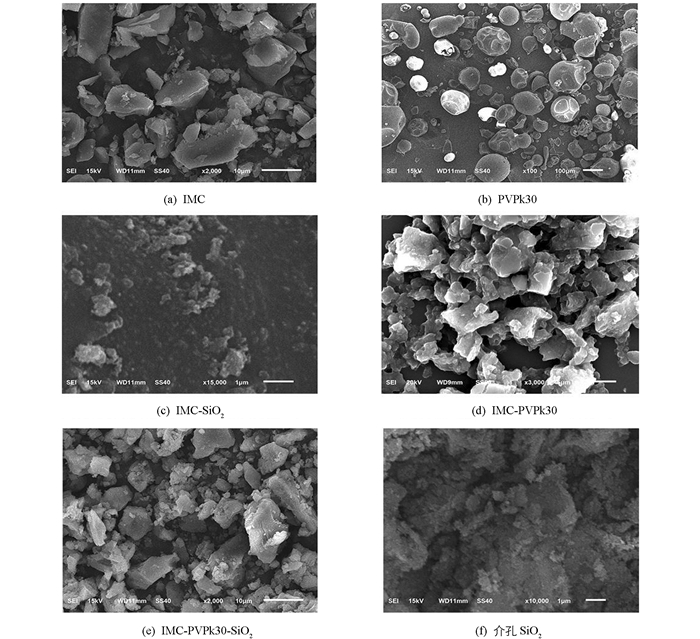
如图 3所示,IMC粉末以规则的片状或柱状结构晶体存在,结构完整均一,由于晶体结构比表面积小,根据Noyes-Whitney方程得知溶出率低. PVPk30呈圆球状,与IMC制备成IMC-PVPk30固体分散体后,结构呈不规则块状. IMC以微晶或无定形态分散于PVPk30中,增大了药物的比表面积和与介质的接触面积,从而使得其溶解度、溶出度和溶出率得到改善. SiO2呈疏松块状结构,制备成IMC-SiO2和IMC-PVPk30-SiO2后,粒径无明显变化,IMC以微晶或无定形态分散于SiO2的孔道内表面或外表面,进一步增大了药物的比表面积和与介质的接触面积,从而有可能使得其溶解度和溶出度得到一定程度的提升.
-
如表 1所示,利用浸没法制备的IMC-PVPk30,IMC的载药率较低,而在用浸没-溶剂蒸发法制备的IMC-SiO2和IMC-PVPk30-SiO2中,IMC载药率与理论载药率基本一致,这可能是因为SiO2的比表面积和比孔容大,能在表面和孔道内负载较多的IMC.
-
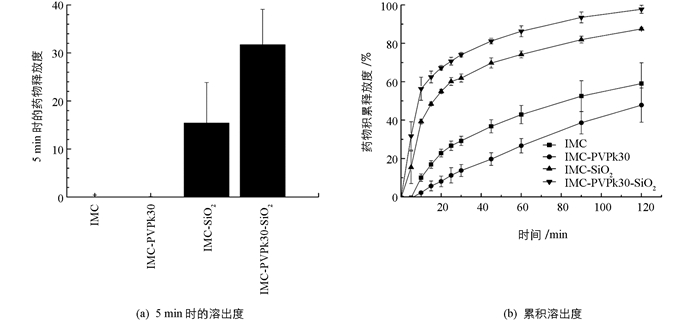
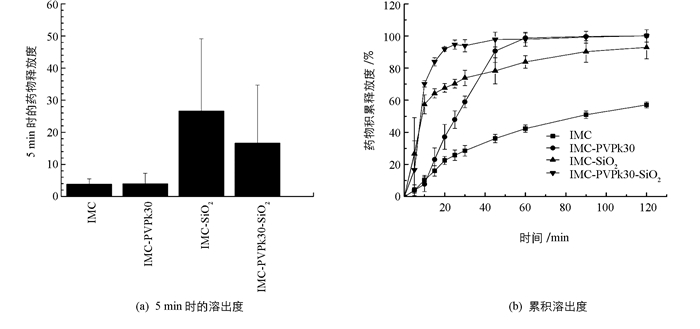
图 4和图 5分别是固体分散体及复合物样品在磷酸盐缓冲液(pH值为7.2)与水的比例为1:4的溶液和人工肠液中的累积溶出度.由图可知,IMC原料和IMC-SiO2,IMC-PVPk30,IMC-PVPk30-SiO2在不同的溶出介质中,IMC的溶出曲线差异较大.
由图 4a可知,在磷酸盐缓冲液(pH值为7.2)与水的比例为1:4的溶液中,IMC原料和IMC-PVPk30在5 min时未检测到IMC的释放,而IMC-SiO2在5 min溶出约15%,IMC-PVPk30-SiO2在5 min溶出约32%,其释放速率明显提高.由图 4b可知,在120 min内,IMC原料溶出缓慢,且120 min时约有58%的药物释放.而IMC-PVPk30的溶出率低于IMC原料,可能原因为,一是固体分散体中药物的溶出需要历经分散体的溶蚀与微晶的溶解两个过程;二是固体分散体中的PVPk30吸水膨胀阻碍了IMC的释放,从而导致IMC释放速率降低.与IMC-PVPk30相比,IMC-SiO2的溶出率显著提高,可能因为介孔SiO2其亲水性及较高的比表面积增加了固体分散体的润湿性,加快了IMC在溶出介质的释放.而与IMC-SiO2相比,IMC-PVPk30-SiO2的溶出率也有所提高,这可能是IMC,PVPk30,SiO2之间相互作用而引起的.
由图 5可知,在人工肠液中,IMC和IMC-PVPk30于5 min时的溶出度均较低(小于4%),但5 min后IMC-PVPk30的溶出率显著提高,远大于IMC,在60 min时药物已完全释放到介质中. IMC原料药的溶出缓慢,在120 min仅释放50%左右,而IMC-SiO2的溶出率较原料药明显提高,120 min药物释放90%左右,但并未释放完全.相比于其他组,IMC-PVPk30-SiO2的溶出率高于IMC-SiO2和IMC-PVPk30,40 min左右药物完全释放到介质中,而IMC-PVPk30-SiO2在磷酸盐缓冲液中120 min药物释放95%左右,表明将介孔SiO2和PVPk30共同作为IMC的载体材料,可显著提高难溶性IMC的溶出度.
2.1. 制备所得的固体分散体
2.1.1. DSC测定结果
2.1.2. SEM观察结果
2.2. 载药率测定
2.3. 溶出度测定结果
-
利用改良后的浸没-溶剂蒸发法制备的介孔二氧化硅吲哚美辛固体分散体复合物(IMC-PVPk30-SiO2),具有较高的载药率和溶出率,有望成为难溶性药物的新型递药系统.







 DownLoad:
DownLoad: