-
月季切花作为世界切花产业的重要组成部分,年贸易额可达全球切花总贸易额的30%以上[1].然而,在其采后流通过程中,极易遭受外源乙烯、失水胁迫等不利因素影响,引起花朵开放异常(僵花、僵蕾、提前衰老等),造成巨大的采后损耗[2].因此,如何延长月季切花采后品质已成为国际研究热点.
月季花朵衰老受外源植物激素调控,其中乙烯(C2H4)与脱落酸(ABA)促进衰老,细胞分裂素(CTKs)与赤霉素(GAs)延缓衰老[3].通过比较不同月季品种的内源细胞分裂素含量发现,月季切花采后瓶插寿命与内源细胞分裂素含量成正相关,细胞分裂素含量越高的月季品种,往往具有更长的瓶插寿命[4].外源细胞分裂素处理可有效延长月季、香石竹、矮牵牛等切花的采后瓶插寿命[5];此外,应用6-甲基嘌呤(一种细胞分裂素脱氢酶/氧化酶抑制剂)也能有效延缓花朵衰老[6].与之相反,在香石竹衰老的花瓣中发现,2个编码细胞分裂素氧化酶/脱氢酶基因的转录本被显著诱导[7].
细胞分裂素信号转导途径首次报道至今,其转导机制已逐渐被阐明[8].作为细胞分裂素信号转导途径中的重要组成部分,拟南芥的22个响应调节因子(Response Regulators / RRs)基于氨基酸序列相似程度,被划分为A,B两大类.其中,ARR3-9,15-17属于A类RRs;ARR1-2,10-14,18-21属于B类RRs[9].研究发现,RRs基因广泛参与植物生长发育调控.例如,拟南芥A类RRs基因ARR4可通过改变红光敏感性进而参与了植物发育调节[10].在根部具有高表达丰度的A类狗蔷薇RRs基因RcRR1,通过减少外植体的细胞分裂素敏感性以及改变生长素分布,在细胞分裂素诱导的器官再生中发挥了重要作用[11].一个杨树的B类RRs基因PtRR13,通过改变维管形成负调节基因CONTINUOUS VASCULAR RING1、生长素转运子基因PLEIOTROPIC DRUG RESISTANCE TRANSPORTER9以及2个乙烯响应基因APETALA2/ETHYLENE RESPONSE FACTOR的表达量,进而负调控杨树不定根发育[12].拟南芥B类RRs基因ARR1,ARR10与ARR12三重突变体研究发现,ARR1,ARR10,ARR12共同通过维持细胞膜完整性、增强色素合成、提高脱落酸(ABA)敏感性以及减少气孔导度进而负调控植物的干旱耐性[13].此外,ARR2基因过表达拟南芥植株表现出促进主根伸长、延缓叶片衰老等表型[14].
然而,迄今为止,关于月季RhRRs基因是否参与了花朵衰老调控仍然未知.本研究以切花月季Samantha为试材,明确了细胞分裂素调控月季切花采后瓶插寿命的作用规律;获得2个受月季花朵衰老显著调节的RhRRs基因RU39694与RU12149;克隆RU39694基因全长序列,进行同源基因的蛋白序列比对及进化树分析,并检测该基因对不同植物激素的应答特性.旨在为解析RhRRs基因调控月季花朵衰老奠定基础.
HTML
-
试验在中国农业大学进行.月季切花Rosa hybrida Samantha采自中国农业大学花圃.采摘标准参考Ma等[15]2005年制定的标准.
植物激素处理:植物激素浓度参考Lyu等[16]的用量标准.将平衡2 h后且无病害的Stage 2月季切花分别置于含有GA3 100 μmol/L、6-BA 100 μmol/L以及ABA 100 μmol/L的处理液中24 h,对照组仅为SDW.处理结束后,将月季切花转移至设定好的环境中瓶插,环境温度(22±2) ℃,相对湿度60%,1 d中光照设置16 h、黑暗8 h.
乙烯处理:将月季切花置于密闭的64 L玻璃箱内,注入浓度为10 μmol/L乙烯气体,处理24 h.为防止CO2积累,需在箱内放置1 mol/L NaOH溶液.对照组玻璃箱内无乙烯.
-
分别摘取月季花朵的第3层花瓣,液氮速冻后备用.月季花瓣总RNA提取参考Hot Borate法[17],并做部分修改.反转录采用Vazyme公司HiScript© Ⅱ Q Select RT Supermix for qPCR试剂盒,反应条件为:25 ℃ 10 min;50 ℃ 30 s,85 ℃ 5 min. RT-PCR检测采用CWBIO公司的2×Taq Master Mix试剂盒,反应条件为:95 ℃ 5 min;95 ℃ 30 s,60 ℃ 30 s,72 ℃ 1 min (循环);72 ℃ 10 min.月季RhActin5为内参基因. PCR产物用1.5%的琼脂糖凝胶进行分离.引物及其序列见表 1.
-
基因克隆参考Wu等[18]的方法,并稍作修改.
RhRR9基因3'端克隆:通过实验室转录组数据库(http://bioinfo.bti.cornell.edu/rose)提供的序列,设计特异上游引物RhRR9-F1,F2,分别与3'-RACE试剂盒提供的2条下游引物进行2轮巢式PCR.
RhRR9基因5'端克隆:根据已获得的RhRR9基因序列,设计两条特异下游反向引物RhRR9-R1,R2,利用5'-RACE kit试剂盒提供的5' RACE outer primer及5' RACE inner primer进行2轮巢式PCR.
扩增产物采用Takara公司DNA凝胶回收试剂盒回收,连接采用pGEM T-Easy vector,转化采用大肠杆菌DH5α,经蓝白斑筛选后,阳性克隆送至三博远志公司测序.
-
通过DNAMAN 5.0将RhRR9基因全长ORF序列翻译成氨基酸,并利用ProtParam(http://web.expasy.org/protparam)分析其蛋白特性. RhRR9蛋白同源序列分析采用Clustal及BioEdit,系统进化树分析采用MEGA 5.0.
1.1. 试验材料和植物激素处理
1.2. 总RNA提取与RT-PCR检测
1.3. 基因克隆
1.4. 蛋白特性及系统进化树分析
-
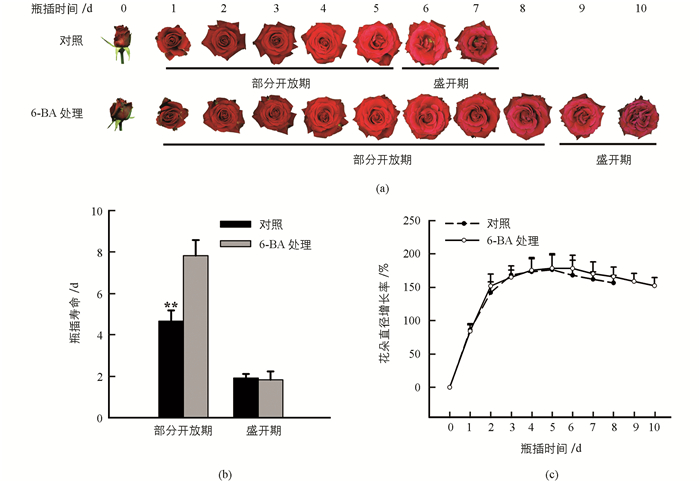
根据Ma等[15]以及Wu等[18]的研究结果,月季切花采后品质分为3个阶段,2~4级为部分开放期(Partially opened stage),5级为盛开期(Fully opened stage),6级为衰老期(Senescent stage).本试验以2级月季切花为试材,分别观察了外源细胞分裂素处理对月季切花采后品质保持的作用效果(图 1).如图 1a,1b所示,细胞分裂素处理能显著延长月季切花瓶插寿命,其作用阶段主要在部分开放期(Stage 2~4),瓶插天数由对照组的4.67 d延长至7.83 d;而盛开期(Stage 5)并无显著差异,处理组和对照组分别为1.83 d与1.91 d.此外研究表明,外源细胞分裂素处理对月季切花花朵直径增长率无显著影响(图 1c).
-
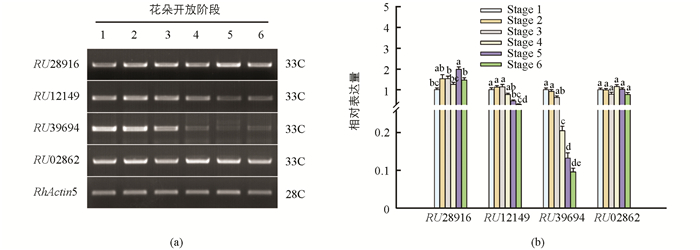
为获得响应月季花朵衰老的RhRRs基因,从实验室月季花朵发育转录组数据库(http://bioinfo.bti.cornell.edu/cgi-bin/rose_454/index.cgi)中共筛选获得了4条注释为RhRRs的EST序列,分别为:RU28916,RU12149,RU39694以及RU02862.根据已知序列分别设计特异引物,利用RT-PCR方法对其在月季花朵开放和衰老(stage 1-6)进程中的表达谱进行了检测(图 2a),并运用AlphalmagerTM2200软件进行了数据量化(图 2B).如图 2所示,RU28916与RU02862两个基因的表达量在月季花朵开放和衰老进程中均无显著变化;RU12149基因从stage 5开始呈显著下降趋势,到stage 6时其表达量为Stage 4级的1/3;RU39694基因在花朵部分开放阶段(Stage 1~3)保持了较高的表达水平,且没有显著差异,然而从stage 4开始急剧下降,到stage 6时其表达量仅为Stage 3的1/10;推测RU39694与RU12149基因可能参与了月季花朵衰老进程.基于4个RhRRs基因在月季花朵不同开放阶段的表达谱,选择在月季花朵采后衰老进程中表达变化最为显著的RU39694作为候选基因进行进一步研究.
-
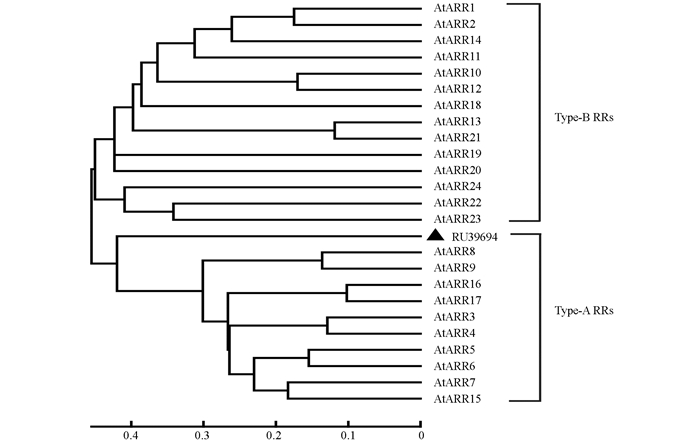
通过RACE PCR方法克隆得到RU39694基因全1 292 bp,其中含有258 bp 5'-UTR,296 bp 3'-UTR,ORF序列738 bp,共编码245个氨基酸序列;将其与拟南芥22个RRs蛋白序列进行系统进化树分析发现(图 3),RU39694蛋白属于A类RRs蛋白,且与拟南芥ARR8以及ARR9亲缘关系最近,因此命名为RhRR9.
-
通过ProtParam分析发现,RhRR9蛋白分子式为C1176H1912N324O401S12,分子量为27.39 kDa,半衰期在酵母(Yeast)中大于20 h,大肠杆菌(Escherichia coli)中大于10 h,亲水性系数(GRAVY)为-0.613,为疏水性蛋白;RhRR9蛋白肽链负电荷残基(Asp + Glu)为39,正电荷残基(Arg + Lys)为28,推定的等电点为4.88.
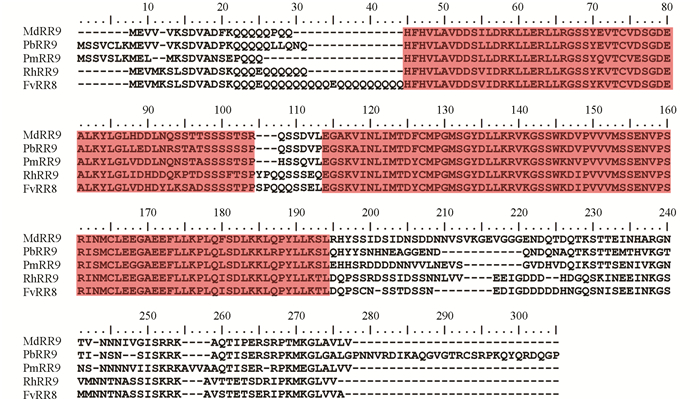
通过与草莓(Fragaria vesca)、梅花(Prunus mume)、苹果(Malus domestica)、梨(Pyrus x bretschneideri)等同源基因氨基酸序列比对发现(图 4),RhRR9序列含有A类RRs基因特有保守结构域,含有“DDK”基序,同时序列中间存在11~21个不同的氨基酸残基,且蛋白序列C端均不相同.
-
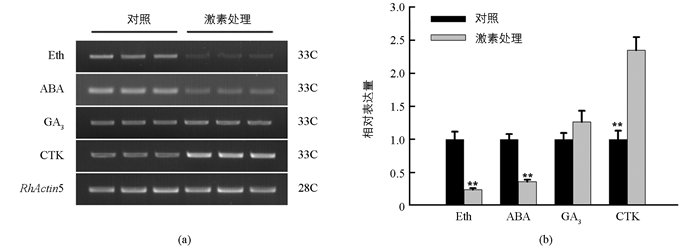
为进一步明确RhRR9基因的植物激素诱导特性,利用RT-PCR方法检测了不同植物激素处理后的表达谱(图 5a),并运用AlphalmagerTM 2200软件进行了量化(图 5b).结果显示,RhRR9基因表达谱受外源植物激素中的细胞分裂素显著诱导,而被ABA、乙烯显著抑制,但其表达量不受外源赤霉素的影响.
2.1. 外源细胞分裂素处理对月季切花采后品质保持的作用效果
2.2. 响应月季花朵衰老的RhRRs基因筛选
2.3. RU39694基因全长克隆及系统进化树分析
2.4. RhRR9蛋白特性及同源序列比对分析
2.5. RhRR9基因的植物激素诱导特性分析
-
外源细胞分裂素处理可有效延长月季切花采后瓶插寿命[19],主要通过减少月季切花离子渗透率,增强抗氧化物酶、过氧化氢酶以及过氧化物酶活性[20].在分子水平,RhHB6-RhPR10.1模块通过调控月季花朵内源细胞分裂素含量进而拮抗乙烯诱导的月季花朵衰老[18];最新研究表明,RhERF113也通过改变月季花朵内源细胞分裂素含量参与了月季花朵衰老进程[21].然而,外源细胞分裂素处理在月季切花采后哪一阶段发挥作用,及其是否影响花径增长率仍然未知.本试验以月季Samantha为试材,系统研究了外源细胞分裂素处理对月季切花采后品质保持的作用效果以及作用规律.结果表明:外源细胞分裂素处理可有效延长月季切花采后瓶插寿命,作用效果主要体现在花朵部分开放期(Stage 2-4),由对照的4.67 d延长至7.83 d,而花朵盛开期(Stage 5)无显著差异;此外,细胞分裂素处理对月季切花采后花朵直径增长率无显著效果.
作为细胞分裂素信号转导途径的重要组成部分,越来越多的响应调节因子(Response regulators)基于其特有的蛋白结构特性从不同物种中被鉴定,如拟南芥[13]、狗蔷薇[11]、杨树[12]等.研究发现,RRs基因可能参与植物衰老调控;例如,拟南芥ARR5基因的表达量受叶片衰老显著抑制[22];过表达ARR2的拟南芥叶片的衰老被延迟[14].为筛选响应月季花朵衰老的RhRRs基因,本研究从月季转录组数据中得到4条RhRRs基因的EST序列,并对其在月季花朵开放进程中的表达谱进行了检测.其中RU39694基因的表达谱受月季花朵衰老显著抑制;其全长ORF(开放阅读框)为738 bp,编码245个氨基酸序列,含有A类RRs基因特有的“DDK”保守基序,属于A类RRs家族基因;系统进化树分析表明,该蛋白与拟南芥AtARR8,AtARR9亲缘关系最近.同时,RhRR9基因的表达谱受外源细胞分裂素处理显著诱导;该表达特性与前人报道的A类RRs基因表达谱受外源细胞分裂素处理显著诱导相一致[9].此外,RhRR9基因表达谱可被外源ABA和乙烯处理显著抑制,可能与ABA、乙烯处理加速月季衰老有关[16, 18].
本研究明确了外源细胞分裂素处理延长月季切花采后品质期的主要作用阶段,获得了一个响应月季花朵衰老且被外源细胞分裂素显著诱导、ABA与乙烯显著抑制的RhRR9基因.该结果为解析RhRRs基因在调控月季花朵衰老中的作用机理提供了参考,但RhRR9基因的生物学功能仍需进一步阐明.






 DownLoad:
DownLoad: