-
对人体而言,铅是多系统、多亲和性的毒物,是不可生物降解的累积毒物,可在肾脏、肝脏、骨骼和大脑等多个身体器官中缓慢积累,即使长期低水平铅暴露也会对人类健康造成极大的伤害[1-2].铅离子主要来源于金属回收(再生铅),锡箔加工、冶炼和蓄电池制造等工业活动中.为了治理重金属污染,人们采用了很多方法,如化学沉淀、离子交换、超滤、反渗透和电渗析等[3].由于技术或经济上的限制,传统的水处理程序通常不适合从地表水中有效去除低水平的重金属.而对于某些类型的生物质,即使是非常稀的水溶液,也能够结合和浓缩金属,因此生物吸附法从水溶液中去除和回收重金属越来越受到重视.
蛋壳膜表面含有大量的氨基以及羟基、羧基、二硫键胱氨酸残基、酰胺基等多种官能团,已用于多种污染物的去除,但吸附量较低,功能化可有效提高其吸附性能.目前已有采用氨基化[4-5]、酸处理[6]、巯基化[7-8]和磺酸化[9]等多种化学改性方法来提高其吸附能力.黄原酸酯是一种含极性基团和非极性基团的阴离子捕收剂,易于制备和金属配合物的高稳定性(低溶解性)产物,在捕收剂中得到广泛应用,加入蛋壳膜可提高蛋壳膜的硫基含量[10-11].本研究旨在通过黄原酸功能化蛋壳膜,并研究其去除水溶液中Pb(Ⅱ)的性能.
HTML
-
电感耦合等离子体原子发射光谱仪(ICP-2060T,江苏天瑞仪器股份有限公司),扫描电镜(KYKY-3200,北京中科科仪技术发展有限公司),傅立叶变换红外光谱仪(以KBr压片测定).超纯水仪(LD-50G-E,重庆利迪实验仪器设备有限公司),恒温振荡培养箱.
蛋壳膜(eggshell membrane,ESM):用自来水冲洗后剥下ESM,用超纯水洗净,放入0.5 mol/L HCl溶液中浸泡去除表面残存钙质,用超纯水洗至中性,煮沸30 min,再用超纯水洗净后于80 ℃烘干,剪碎即得. Pb标准储备溶液的质量浓度为1 000 mg/L.其余试剂均采用分析纯.
-
将1 g ESM浸入50 mL 3.5 mol/L的氢氧化钠中,室温下搅拌3 h,加入2 mL二硫化碳溶液,搅拌3 h,用超纯水洗至中性后,再用V(乙醇):V(水)为70:30的溶液洗涤3次,最后再用无水乙醇洗涤1次,在室温下干燥24 h,得到黄原酸功能化蛋壳膜(xanthate-modified eggshell membrane,XESM).合成机理如化学反应方程式(1),其中R代表蛋壳膜基体.
为了研究黄原酸功能化前后ESM的表面结构及官能团的变化,分别用扫描电镜和红外光谱仪对ESM和XESM进行表征.
-
准确移取50 mL一定pH值的Pb(Ⅱ)溶液至100 mL具塞锥形瓶中,加入50 mg XESM后,置于一定温度下的恒温摇床中,振摇一段时间后(转速为180 r/min),测定上清液中Pb(Ⅱ)的质量浓度,计算XESM对Pb(Ⅱ)的吸附量.实验考察了pH值、吸附时间、共存物质对XESM去除Pb(Ⅱ)的影响.所有实验均进行3次平行实验,结果取其平均值,误差线表示标准差. Pb(Ⅱ)的吸附量q的计算公式为:
式中,q为吸附量(mg/g),V为溶液体积(L),C0为吸附前溶液中Pb(Ⅱ)质量浓度(mg/L),Ce为吸附平衡后溶液中Pb(Ⅱ)质量浓度(mg/L),m为吸附剂的质量(g).
Pb(Ⅱ)浓度采用电感耦合等离子体原子发射光谱仪测定,仪器工作参数如下:高频发生器功率,900 W;等离子气流量,12 L/min;辅助气流量,0.6 L/min;载气流速,0.6 L/min;泵速,1.5 mL/min;积分时间,3 s;读数延迟,30 s.
1.1. 仪器与试剂
1.2. 材料制备及表征
1.3. 吸附实验
-
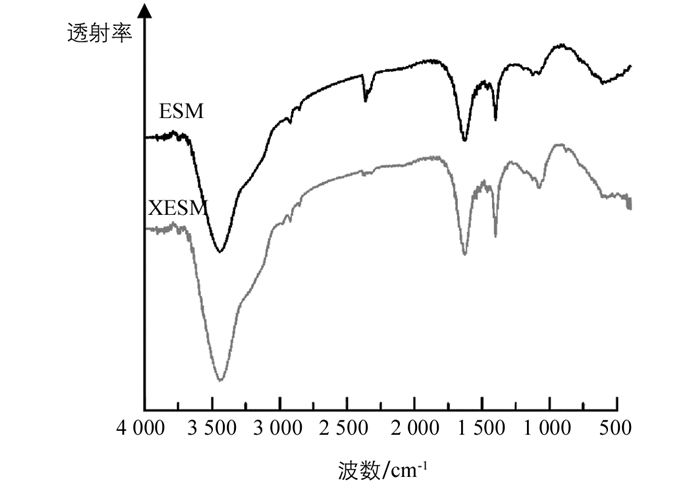
为探究黄原酸功能化前后ESM的官能团变化情况,利用红外光谱仪对ESM和XESM进行分析,结果见图 1.
如图 1所示,通过对黄原酸化修饰前后的ESM进行红外光谱分析,发现ESM的主要官能团化学键所对应的吸收峰强度都有所增加,如3 200~3 600 cm-1的吸收归属于N-H和O-H伸缩振动、1 631 cm-1处的N-H弯曲振动、1 350~1 410 cm-1的吸收归属于C-N伸缩振动和C=O伸缩振动、2 920 cm-1属于饱和C-H键的伸缩振动[4, 12],这可能是因为碱性条件下,部分蛋白质水解,从而使末端官能团增加.此外,黄原酸盐极性基团的主要特征吸收带位于800~1 200 cm-1区域[13].显然,与ESM相比,XESM更强烈. XESM在1 049 cm-1处出现m C=S的峰,C-S-S对称拉伸的峰在1 076 cm-1处.在517 cm-1附近也观察到C-S的非常微弱的振动,表明已成功制备XESM.
-
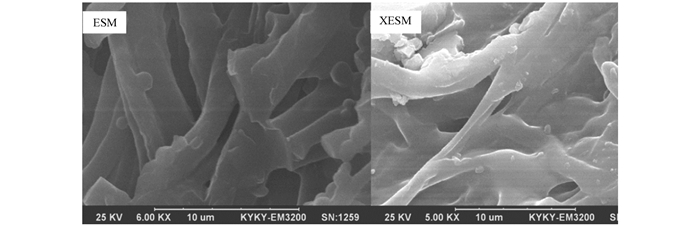
为了进一步探究黄原酸功能化前后ESM表面结构的变化.用扫描电镜对ESM和XESM进行表征,结果见图 2.
由图 2可以发现,ESM表面为网状,有大量清晰的孔隙结构,且孔隙内壁粗糙、分布不规则,这些形态给ESM用作吸附剂提供了可能性,众多的高度交织的大孔结构对于被吸附目标物在吸附剂中的扩散过程非常有利.而XESM表面的网状结构有部分坍塌,但仍然具有孔结构.坍塌的原因可能是在引入黄原酸官能团的过程中ESM表面被破坏,这与红外光谱图结果一致.
-
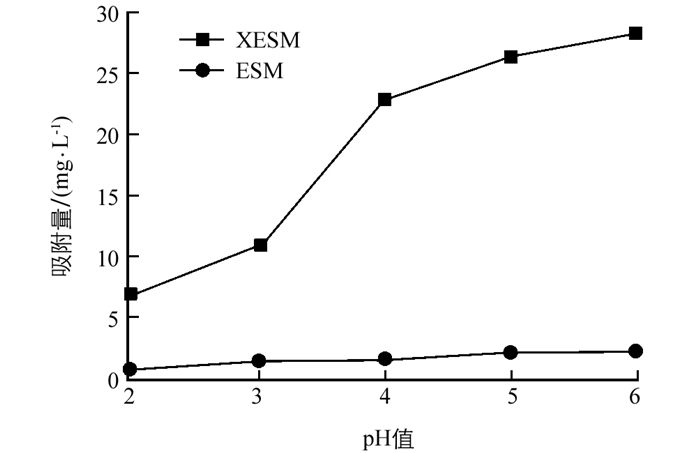
为了考察溶液初始pH值对吸附Pb(Ⅱ)的影响,并排除Pb(Ⅱ)产生沉淀的干扰,实验对pH值2~6范围进行了探究(图 3).
结果表明,随着pH值增大,XESM对Pb(Ⅱ)的吸附量增加明显.这可能是由于低pH值时,H+与Pb(Ⅱ)竞争吸附位点,且黄原酸功能化结构容易被破坏.随着pH值增加,溶液中H+浓度降低,与Pb(Ⅱ)竞争作用降低,Pb(Ⅱ)与XESM表面上带负电荷的吸附位点发生静电引力,使吸附量逐渐增加.而pH值的变化对ESM的吸附性能影响不大.
-
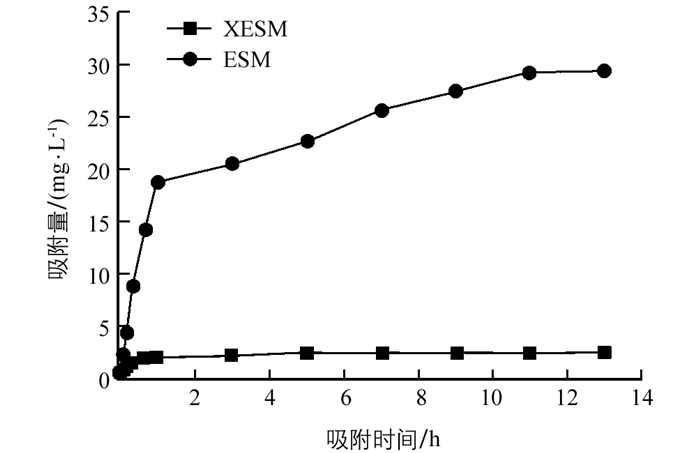
如图 4所示,XESM及ESM对Pb(Ⅱ)的吸附过程呈现出先快速后慢速最终达到动态吸附平衡的趋势. Pb(Ⅱ)在XESM上的吸附过程主要分为3个阶段:吸附开始至1 h内,是快速吸附阶段.从1 h后,为慢速吸附阶段;到11 h后进入动态吸附平衡阶段.可能是在吸附的初始阶段,整个体系中Pb(Ⅱ)质量浓度的均匀分布在吸附剂的表面,因此初期吸附速度快,吸附量增加得很快.随着吸附时间的延长,吸附剂附近的Pb(Ⅱ)浓度下降,远处的铅离子到达吸附剂表面需要的时间也越来越长,吸附量增加缓慢,直至整个吸附过程达到动态平衡. XESM及ESM对Pb(Ⅱ)的饱和吸附量分别为(29.43±0.87)和(2.39±0.02) mg/g,可见黄原酸功能化对ESM吸附铅离子的吸附量影响显著.
采用准一级动力学模型(式2)和准二级动力学模型(式3)[4]分别对XESM及ESM吸附Pb(Ⅱ)的吸附动力学特征进行拟合:
式中,qe表示吸附平衡时的吸附容量(mg/g),t表示吸附的时间(min),qt表示时间t时蛋壳膜上铅离子的吸附量(mg/g),k1表示准一级动力学方程常数(min-1),ν0表示初始吸附速率(mg/(g·min)).
各拟合参数如表 1所示,发现准二级动力学较为符合XESM及ESM对Pb(Ⅱ)的吸附行为,拟合值接近实验平衡时的吸附量,且相关系数较高(r2>0.99).这表明吸附过程是以吸附剂表面的功能活性位点主导的化学吸附为主[14].
-
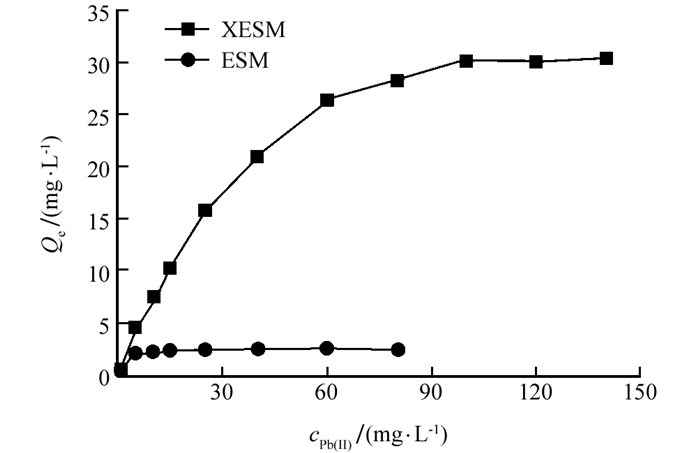
实验考察了30 ℃条件下,XESM及ESM对Pb(Ⅱ)的吸附等温曲线(图 5).吸附量随Pb(Ⅱ)质量浓度的增加而增加.这可能是在Pb(Ⅱ)浓度较低时,吸附剂表面的活性位点相对较多,随着铅离子浓度增加,Pb(Ⅱ)占据的吸附位点增加,当超过吸附剂表面可用吸附位点时,主要发生在吸附剂表面的吸附接近饱和.当Pb(Ⅱ)的质量浓度分别接近5,100 mg/L时,ESM及XESM对Pb(Ⅱ)的吸附趋于饱和,吸附量分别为(2.46±0.52)和(30.48±2.01) mg/g.
分别用Langmuir等温吸附模型和Freundlich等温吸附模型[7]对实验数据进行拟合:
式中,Ce为吸附平衡浓度(mg/L),qe是平衡吸附量(mg/g),qmax是理论最大吸附量(mg/g),b为吸附能相关的Langmuir模型常数(L/mg),k和n是与相对吸附容量和吸附强度相关的Freundlich吸附模型常数.
各拟合参数列于表 2,可知,XESM及ESM对Pb(Ⅱ)的吸附较为符合Langmuir等温方程式,这表明吸附剂可均匀吸附铅离子,但当吸附剂表面的活性位点吸附铅离子后则不再具有持续吸附的活性. Langmuir模型拟合的XESM对Pb(Ⅱ)的最大吸附量为33.11 mg/g,约为ESM对Pb(Ⅱ)吸附量(2.46 mg/g)的13.5倍.
-
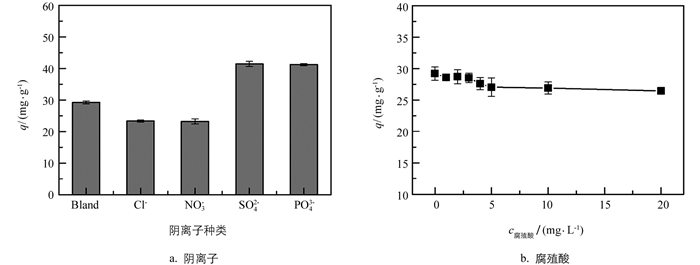
实验考察了水体中一些常见的共存物质,如阳离子(Na+,K+,Ca2+,Mg2+,Zn2+,Al3+和Fe3+)(表 3)、阴离子(Cl-,SO42-,PO43-,NO3-)和自然有机物质(以腐殖酸为代表)等对吸附体系的影响.
从表 3可知,Na+,K+的质量浓度远高于Pb(Ⅱ)的质量浓度,达到500 mg/L时,吸附量的降低也不足10%,对吸附体系影响小.价态较高的Ca2+,Mg2+,Zn2+,Al3+和Fe3+,虽与黄原酸盐结合能力较弱,但或多或少会与Pb(Ⅱ)竞争吸附位点,而对吸附Pb(Ⅱ)产生抑制作用.
图 6的结果显示,SO42-和PO43-的存在有利于Pb(Ⅱ)的去除,这是由于吸附和沉淀的双重作用造成的.而Cl-和NO3-对Pb(Ⅱ)吸附的抑制作用不超过20%.鉴于天然水样中共存阴离子的真实浓度远远低于实验中的浓度,因此共存阴离子对吸附能力的影响可以忽略[15].另外,随着腐殖酸浓度的升高,XESM对Pb(Ⅱ)的吸附量略有降低,这可能是由于水中的有机质会覆在吸附材料表面,从而抑制了对水中Pb(Ⅱ)的去除能力.






 DownLoad:
DownLoad: