-
开放科学(资源服务)标志码(OSID):

-
滞育是生物体发育到某一特定阶段停滞生长发育来抵抗外界不利环境的一种生理现象,普遍发生在线虫、昆虫、鱼类甚至哺乳动物上[1-5]. 当生物体受到光照、温度等外界信号诱导时,机体会发生一系列变化:呼吸减弱、代谢降低、抗逆性增强、发育逐渐停滞进入滞育. 不同于休眠,滞育通常发生在不利条件来临前,在滞育过程中即使适宜的条件到来也不会立即打破滞育,需要经历一定时间或受到某一因子刺激后,生物体才能解除滞育. 家蚕是典型的卵滞育昆虫,在胚胎早期进入滞育[6],根据滞育程度的差异可以划分为一化性品种,自然条件下1年只孵化1次;二化性品种,自然条件下1年只孵化2次;多化性品种,自然条件下1年孵化多次. 其中,二化性家蚕可以通过对其母代家蚕催青时期条件进行设置来控制子代家蚕的滞育性[7],即高温长光照催青获得子代滞育卵,低温黑暗获得子代非滞育卵.
热激蛋白(heat shock proteins,Hsps)是一种高度保守的蛋白,可防止底物蛋白的不可逆变性,从而增强生物体的抗逆性,广泛存在于各种原核和真核生物中. 根据Hsps的分子量和同源关系可以分为4种[8],分别为Hsp90,Hsp70,Hsp60和sHsps(small Hsps,sHsps). 其中,sHsps的相对分子质量在1.2×104~4.2×104之间,通常在3.0×104以下,有一个α-晶体区域包含约100个氨基酸残基的保守序列[8-9]. Hsps最初是在果蝇的研究中被发现[10],是对高温有应激反应的一种蛋白,并因此得名. 随后的研究发现,Hsps可以在寒冷、干燥、缺氧和化学药品等应激条件下上调表达[11].
热激蛋白广泛参与到生物生长发育的调控过程中. 在昆虫滞育过程中,麦红吸浆虫Sitodiplosis mosellana的Hsp70和Hsp90在幼虫滞育和一定范围的冷热胁迫下表达上调,而Hsp70在滞育之前和滞育期间丰度相当[11];蛀茎夜蛾Sesamia nonagrioides滞育幼虫中Hsp90的表达量增加,而Hsp70在滞育过程中减少[13];非洲棉铃虫Helicoverpa armigera滞育期间脑中Hsp20.7和Hsp90的表达量下降,而Hsp21.4和Hsp70表达量升高[14]. 热激蛋白在滞育过程中含量的变化,暗示其可能在当中发挥了重要作用,在滞育过程和应急反应时可能具有相似功能.
目前,有多个家蚕Hsps被研究鉴定:Fan等[15]采用鸟枪蛋白组学法对蚕卵进行研究,发现了9个Hsp蛋白;Li等[16]通过全基因组分析鉴定了16个家蚕sHsp基因,并发现昆虫sHSPs显示出与脊椎动物完全不同的进化模式. 在我们前期的转录组学研究中[17],通过对产后32 h的滞育卵和发育蚕卵进行RNASeq测序分析,发现了一些差异表达的家蚕Hsp基因. 本文中我们选取BmHsp19.5基因(BGIBMGA013545)进行进一步分析,完成了克隆鉴定和生物信息学分析,并对该基因在滞育卵和非滞育卵中产后时期的表达情况进行了调查分析. 这些结果将为揭示家蚕滞育过程中Hsps基因的表达特征及功能提供重要的研究信息,也为研究其他昆虫的Hsps提供有价值的参考.
HTML
-
本研究使用的家蚕二化性品种‘大造’由西南大学家蚕基因资源库提供,母代家蚕通过高温25 ℃、长光照条件下催青产下滞育卵,低温15 ℃、短日照条件下催青产下非滞育卵为实验材料. 按照常规方法饲养家蚕,取子代蚕卵作为实验材料. 将蚕卵置于1.5 mL离心管中,用液氮速冻,保存于-80 ℃冰箱备用.
-
根据前期转录组测序结果获得的家蚕BmHsp19.5基因序列,通过家蚕基因组数据库Silkdb 3.0(https://silkworm.swu.edu.cn/silkdb/) Blast检索,采用Primer 5.0软件在预测序列上设计克隆引物. 基因鉴定后,为调查BmHsp19.5基因在产后时期的表达情况设计了qPCR引物序列(表 1).
-
PCR循环参数为:94 ℃预变性1.5 min;94 ℃变性20 s,58 ℃退火30 s,72 ℃延伸40 s,28个循环;72 ℃延伸10 min. 扩增产物与pMD19-T载体连接,转化进入E.coli DH5α感受态细胞中,涂布含有氨苄抗性的平板筛选后,送至上海生工生物工程有限公司进行测序鉴定.
-
利用ProtParm在线程序(https://www.expasy.org/tools/protparam.html)分析家蚕BmHsp19.5蛋白理化参数;应用Mega 7.0软件、BLAST(https://blast.ncbi.nlm.nih.gov/Blast.cgi)和(https://silkworm.swu.edu.cn/silkdb/)程序对BmHsp19.5蛋白序列做同源性比较,绘制系统进化树. 采用SignalP在线程序(https://www.cbs.dtu.dk/services/SignalP-4.1/)分析BmHsp19.5蛋白信号肽的功能;使用TMHMM在线程序(https://www.cbs.dtu.dk/services/TMHMM/)和Expasy Protscale在线程序(https://web.expasy.orgprotscale/l)进行BmHsp19.5蛋白质跨膜区预测和疏水性分析;使用SMART软件(https://smart.embl-heidelberg.de/)对蛋白功能结构域预测.
-
qPCR采用ABI Step One仪器进行两步法反应. 反应程序为:94 ℃预变性1 min,94 ℃变性20 s,60 ℃退火延伸40 s,30个循环. 每个时期设置3个生物学重复,每个生物学重复设置3个技术重复,根据荧光定量所得到的Ct值,采用2-ΔΔCt法对数据进行分析,GraphPad Prism 7.0软件作图.
-
数据结果用平均值±标准差表示,使用GraphPadPrism 5.0软件进行统计分析,同时采用多重T-test方法来比较不同样品的差异具有统计学意义(p<0.05).
1.1. 实验材料
1.2. 引物设计
1.3. 基因克隆
1.4. BmHsp19.5生物信息学分析
1.5. 家蚕BmHsp19.5在蚕卵产后不同时期的检测
1.6. 数据统计学分析
-
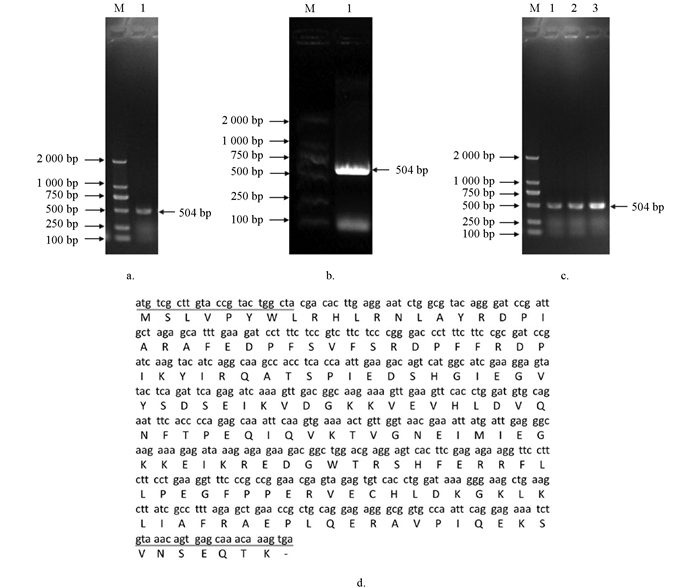
目的基因以家蚕产下32 h的蚕卵cDNA为模板,用克隆引物进行PCR扩增,随后用琼脂糖凝胶电泳检验(图 1a),结果显示PCR产物大小在504 bp,与预测编码区序列大小一致. 随后进行凝胶回收并验证(图 1b),并将回收片段连接到载体上(图 1c). 测序结果在Silkdb 3.0网站比对分析后,表明BmHsp19.5基因没有内含子,全长为732 bp,其中ORF为504 bp,编码的氨基酸序列为167个氨基酸(图 1d),与预测序列结果一致.
-
利用Protparam软件对家蚕BmHsp19.5基因所编码的目的蛋白进行预测分析,其理论相对分子质量为1.95×104,等电点为5.21. 不稳定指数(II)为38.29,说明蛋白质稳定. 利用SignalP 4.1Server软件分析预测BmHsp19.5蛋白的信号肽,结果显示未发现有信号肽结构,可能该目的蛋白为非分泌蛋白. 利用TMHMM在线预测网站预测家蚕BmHsp19.5蛋白跨膜结构域,可知该蛋白不具有跨膜结构域,为非跨膜蛋白.
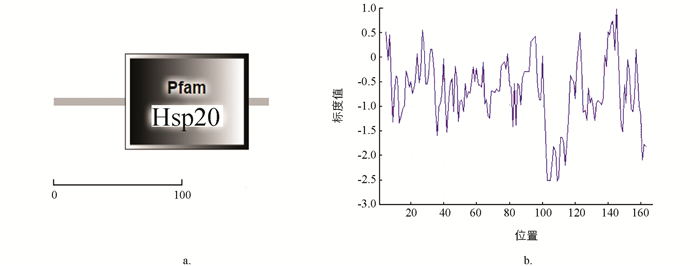
利用SMART软件对BmHsp19.5蛋白进行功能结构域预测,发现该目的蛋白65-157位氨基酸为Hsp20功能结构域(图 2a),故该蛋白属于小分子热激蛋白家族. 利用ProtScale软件对家蚕BmHsp19.5蛋白进行蛋白亲疏水性预测(图 2b),图 2b中横坐标表示氨基酸序列位置;纵坐标表示氨基酸的标度值;通过标度值判定氨基酸的亲疏水性,标度值大于0表示疏水性,标度值小于0表示亲水性. 从图 2b中可知最低值为-2.522,最高值为0.989,且大部分峰值位于0值以下,该目的蛋白为亲水性蛋白.
-
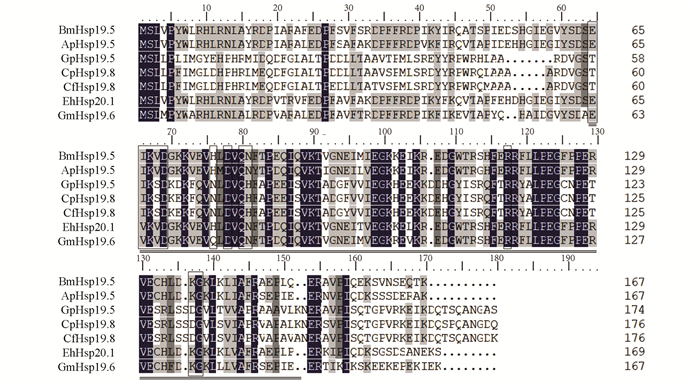
使用家蚕BmHsp19.5蛋白序列在NCBI(National center for biotechnology information)数据库进行BlastP搜索,检索发现多个鳞翅目的同源物,最终选择6个具有代表性的序列进行同源序列比对分析. 结果发现,BmHsp19.5与柞蚕ApHsp19.5(GenBank登录号:APX61064.1)、小木蠹蛾EhHsp20.1(GenBank登录号:AYA93247.1)和东方果蛾GmHsp19.6(GenBank登录号:AKS40076.1)的氨基酸序列相似性较高,分别为94%,93%和92%,同源性分别为83%,85%和79%. BmHsp19.5与桑螟GpHsp19.5(GenBank登录号:QGZ00457.1)、云杉芽虫CfHsp19.8(GenBank登录号:AAZ14792.1)和苹果蠹蛾CpHsp19.8(GenBank登录号:ADX96000.1)的氨基酸序列相似性分别为62%,50%和50%,同源性分别为48%,39%和38%. 所有氨基酸序列都含有一个典型的α-晶状体蛋白结构域(图 3).
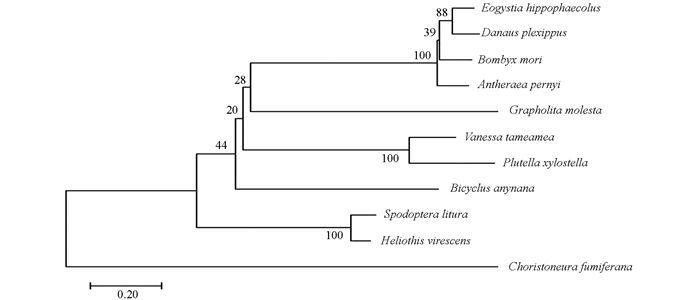
利用Mega 7.0软件对家蚕、柞蚕、小菜夜蛾等11种不同物种的小分子热激蛋白构建系统进化树,结果显示物种序列形成两个大的分支,其中云杉蚜虫单独为一支,其他10个物种序列为一支. 家蚕与黑脉金斑蝶、沙棘蛾这两类首先聚在一起(图 4),然后再与亲缘关系最近的柞蚕聚在一起,之后与梨小食心虫、夏威夷红蛱蝶、小菜蛾等聚为一支.
-
利用qPCR技术检测BmHsp19.5基因在滞育卵与非滞育卵不同时期的表达量(图 5). 在滞育卵中,1~3 d呈上升趋势,在3 d时达到峰值,随后逐渐下降,此时胚胎形态经历了由产后第1 d梨形的头叶分化,到第2 d胚胎明显拉长、尾节分化,在产后第3 d不再有明显变化,整个滞育期保持不变进入滞育Ⅰ期[18]. 通常认为蚕卵在产后2~3 d开始进入滞育期,由此推断BmHsp19.5基因可能在蚕卵进入滞育的准备阶段发挥了重要作用.
从图 5中我们还可以看到,BmHsp19.5基因在滞育卵的表达量显著高于非滞育卵的表达量. 在非滞育卵中产后1 d表达量最高,到第5 d呈持续下将的趋势,在第6 d蚕卵点青的时候表达量又小幅升高,孵化前第8 d略微下降;点青期蚁蚕头部黑色素开始附着,这时蚕体充满了卵壳,为孵化做着最后的准备. 这期间蚕卵需氧量大幅度增加[19],同时活性氧(ROS)含量也增加[20],BmHsp19.5基因的小幅升高似乎与这些变化有关.
2.1. 家蚕BmHsp19.5克隆
2.2. 家蚕BmHsp19.5序列分析
2.3. 家蚕BmHsp19.5同源基因分析判定
2.4. 家蚕BmHsp19.5在蚕卵产后的表达分析
-
根据克隆测序,我们鉴定了一个新的家蚕BmHsp19.5基因,相对分子质量为1.95×104,预测显示该蛋白在65-157位氨基酸为Hsp20功能结构域,符合小热激蛋白家族结构特征. 同源性分析显示该序列与其他鳞翅目昆虫的小热激蛋白具有较高的保守性,都含有一个典型的α-晶体区域. BmHsp19.5基因和大多数sHsp基因一样也只有一个内含子,前人的研究认为由于无内含子就可以避免mRNA剪接过程和当中可能出现的障碍,从时间上优先于其他基因,因此有利于应激或抗逆反应基因的及时表达[21]. 家蚕的sHsp基因sHsp19.9,sHsp20.1,sHsp20.4,sHsp20.8,sHsp21.4和sHsp23.7,仅有含有内含子的sHsp21.4在热激反应中转录物没有增加[22]. 据此我们推测本实验中BmHsp19.5基因可能在应急反应和滞育发育较早时期发挥作用.
热激蛋白的上调似乎是昆虫在不同滞育阶段的一个共同特点. 红尾肉蝇Sarcophaga crassipalpis在蛹滞育期间,大部分Hsp基因会在滞育开始阶段上调表达,并将持续整个越冬期,如Hsp70A,Hsp70B,Hsp60,Hsp25,Hsp23,Hsp18,SmHsp;利用RNAi抑制热激蛋白的表达,发现对蛹的低温存活能力有重要影响[23]. 云杉芽虫中有14种sHsps基因在幼虫进入滞育前、滞育中、滞育后这3个阶段上调表达,如Hsp19.6和Hsp22.1基因在滞育中显著上调表达;Hsp19.2,Hsp19.7,Hsp21.5a和Hsp24.3基因在滞育前和滞育中上调表达;Hsp20.0和Hsp21.3基因则在整个期间持续表达[24]. sHsps常作为细胞防御的第一道防线,在细胞受到胁迫时,作为分子伴侣防止底物蛋白的不可逆变性,反映生物体对环境中存在的某些极端应力的响应[25-26]. 葱蝇Delia antiqua的Hsp23基因在蛹滞育开始时表达量逐渐升高,到滞育维持的中后期达到峰值,在滞育终止时降到较低水平[27]. 在卤虫中由于缺失小热激蛋白p26的胚胎更容易终止滞育,因此认为该蛋白是维持滞育所必需的[28].
家蚕滞育卵室温条件下产后48~72 h开始进入滞育,卵色由淡黄色逐渐变成赤豆色,胚胎形态不再变化,这个时期被称之为前滞育期[29]. 本研究的BmHsp19.5基因在这一时期上调表达,该结果与之前转录组数据一致;根据基因表达模式推测该基因可能参与了滞育这一生理过程,可能在蚕卵进入滞育的准备阶段发挥了重要作用,同时也符合越冬昆虫滞育期间热激蛋白上调以抗寒这个主要特征. 家蚕Hsp20.8A,Hsp70,DnaJ和Hsc71基因也是在滞育蚕卵中上调表达[30]. 也有报道指出家蚕中Hsp20.4,Hsp20.8,Hsp40,Hsp70和Hsp90的mRNA在滞育期间没有变化[31],在家蚕滞育卵和非滞育卵中的含量大致相同[15]. 这可能是由于研究者取材时间的差异所致,同时说明滞育引起的热激蛋白表现和普通应急反应不同,并不是所有热激蛋白都同时参与其中,可能通过滞育相关机制调控Hsp基因及其产物,在不同时间增加、减少或保持不变,从而促进昆虫在逆境中生存[13, 32]. 因此,接下来工作的重点是获得BmHsp19.5较纯蛋白,进一步明确蛋白的功能,并探究其作用机理.







 DownLoad:
DownLoad: