-
开放科学(资源服务)标志码(OSID):

-
犬弓首蛔虫(Toxocara canis)是犬科动物和猫科动物中最普遍的胃肠道寄生线虫,其感染性虫卵进入终末宿主的小肠内可发育为成虫[1-2]. 人类及其他转续宿主摄入则无法完成其生命周期,幼虫在组织内迁移数月或数年,对宿主机体造成严重损害. 人类弓首蛔虫幼虫移行损害的主要器官有肝脏、肺、眼、脑等,引起眼睛和内脏幼虫移行症、神经弓首蛔虫病、隐性弓首蛔虫病等,表现为持续的嗜酸性粒细胞增多,出现视网膜炎、哮喘、癫痫、脑膜炎等症状[3-5],严重危害人类健康.
黏蛋白(Mucins,MUCs)是一类高分子量糖蛋白,其分子由主链和糖基侧链通过O-糖苷键连接组成,主链富含丝氨酸和苏氨酸作为糖基化连接的位点. MUCs包含膜结合型黏蛋白(MUC1,MUC4,MUC16)和分泌型黏蛋白(MUC2,MUC5AC,MUC5B),广泛分布于哺乳动物黏膜表面为其提供保护,并参与细胞分化、粘附、信号转导和免疫反应等多种生物学过程,其异常表达或过表达与肿瘤的发生有关[6-7]. 此外,该蛋白在宿主-寄生虫相互作用的过程中也发挥重要作用. 例如,克氏锥虫的黏蛋白通过与宿主巨噬细胞的L-选择素相结合,抑制白细胞介素-2的产生和T细胞受体相关信号转导蛋白的酪氨酸磷酸化,从而抑制宿主的炎性反应[8];在曼氏血吸虫中,分泌型黏蛋白MUC2形成可脱落的表面涂层,保护寄生虫免受抗体和嗜酸性粒细胞的攻击[9].
目前,对于犬弓首蛔虫黏蛋白2的研究较少. 本研究运用分子生物学技术,首先克隆Tc-muc-2基因并进行序列分析;同时构建Tc-muc-2/pCold TF原核表达载体并制备多克隆抗体,以期为进一步研究Tc-MUC-2的生物学功能奠定基础.
HTML
-
T. canis采自西南大学荣昌校区动物医院的患病犬,鉴定后于液氮中保存.
PremixTaq DNA聚合酶、pMD19-T (simple) Vector、限制性内切酶XhoⅠ、Hind Ⅲ购自TaKaRa公司;琼脂糖凝胶DNA/PCR产物小量回收试剂盒、质粒提取试剂盒购自北京全式金生物技术有限公司;Ni-NTA纯化树脂预装柱、HRP标记的山羊抗兔IgG购自Sangon Biotech公司;Protein A+G Agarose抗体纯化试剂盒购自碧云天生物技术公司.
-
根据T. canis基因组数据(GenBank:AF167707),利用NCBI(National Center for Biotechnology Information)和Primer-Premier对Tc-muc-2进行特异性引物设计(F:5’-CCGCTCGAGATGAACGTTCGTGTCGTCA-3’,引入酶切位点XhoⅠ;R:5’-CCCAAGCTTTTAGCAGAATCCGCAAGTA-3’,引入酶切位点Hind Ⅲ),送至重庆擎科生物科技有限公司进行合成.
-
采用TRIzol法提取T. canis成虫的总RNA,核酸蛋白测定仪检测其浓度和纯度;以提取的总RNA为模板,按反转录试剂盒说明书反转录合成cDNA.
-
以合成的T. canis成虫cDNA为模板进行PCR扩增[10],PCR反应条件为:94 ℃预变性3 min、94 ℃变性30 s、54 ℃退火30 s、72 ℃延伸30 s,共35个循环,最后72 ℃延伸5 min;扩增产物经1%琼脂糖凝胶电泳,用凝胶成像系统扫描并记录结果.
-
按照北京全式金胶回收试剂盒说明书切胶回收PCR产物,按说明书将回收产物与pMD19-T (simple) Vector载体进行连接,全部连接产物转化至100 μL大肠杆菌(Escherichia coli)DH5α感受态细胞中,涂布于含IPTG和X-gal的LB/Amp+琼脂平板中,于37 ℃恒温培养箱正置培养30 min,再倒置培养16 h. 将菌液PCR鉴定为阳性的重组菌液送至重庆擎科兴业生物技术有限公司测序,测序结果利用Clustal Omega和MUSCLE软件进行多重序列比对;用MEGA 5.0邻接法(Neighbour-joining,NJ法)构建系统进化树,并用Bootstrap进行进化树可靠性分析,共1 000个重复.
-
按质粒提取试剂盒说明书提取阳性重组质粒Tc-muc-2/pMD19-T和pCold TF质粒,经限制性内切酶XhoⅠ和Hind Ⅲ双酶切,切胶回收目的基因Tc-muc-2与pCold TF质粒、DNA Ligation Kit进行连接反应. 在冰上配制连接反应液:Digested pCold TF DNA 1 μL,Tc-muc-2 4 μL,Ligation Mix 5 μL;16 ℃反应1 h. 重组表达质粒转化至DH5α感受态细胞,经蓝白斑筛选后,挑取白色单个菌落接种于LB/Amp+液体培养基中,37 ℃,180 r/min培养14~16 h. 提取质粒,进行PCR、双酶切及测序鉴定.
-
挑选阳性菌,提取质粒,并将其转化至Escherichia coli BL21(DE3)感受态细胞,挑单菌落接种于LB/Amp+液体培养基,37 ℃,180 r/min振荡培养6 h,OD600值约为0.8,加入0.8 mmol/L的IPTG 15 ℃诱导24 h;将菌液置于15 ℃,4 500 r/min离心15 min,收集菌体沉淀,用生理盐水洗涤后,再用Washing Buffer重悬菌体,并进行超声破碎,分别取破碎上清液和破碎沉淀进行SDS-PAGE电泳检测;采用Ni-NTA纯化树脂预装柱纯化目的蛋白,利用SDS-PAGE电泳检测纯化结果.
-
利用透析袋去除纯化蛋白中的咪唑,并采用冷冻真空干燥机浓缩蛋白. 将250 μg重组蛋白与弗氏完全佐剂1∶1混合,乳化后以颈背部皮下多点注射的方式对两只健康的新西兰大白兔进行首免;每间隔2周,以200 μg重组蛋白与弗氏不完全佐剂1∶1混合,乳化后进行加强免疫;为激活免疫系统,首免剂量较大,4次免疫后收集血清并检测.
将纯化后的重组蛋白用包被液稀释为5 μg/mL,4 ℃包被12~16 h,PBST(磷酸盐吐温缓冲液)洗3次;加入100 μL BSA(牛血清白蛋白)封闭液37 ℃封闭1 h,PBST洗3次;将获得的兔血清1∶10 000稀释后,进行2倍倍比稀释,免疫前血清作为阴性对照,37 ℃孵育1 h,PBST洗3次;加入HRP(辣根过氧化物酶)标记的山羊抗兔IgG(1∶50 000),37 ℃孵育1 h,PBST洗3次;最后加入3,3′,5,5′-四甲基联苯胺(3,3′,5,5′-Tetra methyl benzidine,TMB)显色液,显色15 min后加入终止液终止反应;酶标仪检测OD450值,当检测孔与阴性孔的比值大于(等于)2.1时的最大稀释倍数为该血清的抗体效价.
达到所需效价后采血制备、纯化多克隆抗体,SDS-PAGE电泳检测抗体纯化结果. 取表达产物进行SDS-PAGE电泳,PVDF(聚偏二氟乙烯)膜平铺至电泳胶,利用转膜缓冲液进行转膜,5 % 脱脂奶粉封闭2 h,TBST(Tris盐吐温缓冲液)清洗3次,兔抗Tc-MUC-2多克隆抗体(1∶10 000)4 ℃孵育12 h,TBST清洗3次,HRP标记的山羊抗兔IgG(1∶8 000)室温孵育12 h,TBST清洗3次,滴加DBA(二氨基联苯胺)反应液,成像.
1.1. 材料
1.2. 方法
1.2.1. 引物的设计与合成
1.2.2. 总RNA的提取与反转录
1.2.3. 目的基因的PCR(聚合酶链反应)扩增
1.2.4. PCR产物的克隆、测序及分析
1.2.5. 原核表达载体的构建
1.2.6. 目的蛋白的表达和纯化
1.2.7. 多克隆抗体的制备及鉴定
-
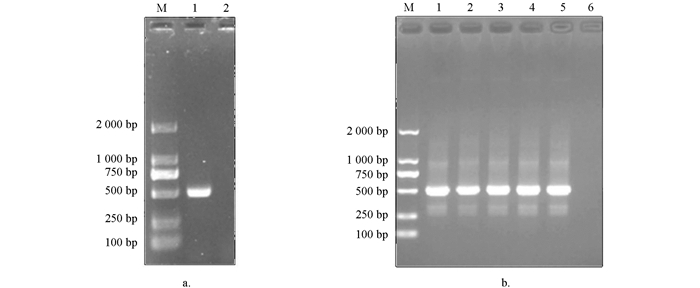
PCR扩增产物经1%琼脂糖凝胶电泳检测,在约549 bp处可见明亮条带,与理论值大小相符;阴性对照没有条带出现(图 1a). 阳性克隆的菌液经PCR扩增后进行1%琼脂糖凝胶电泳检测,结果显示Tc-muc-2/pMD19T在511 bp处有特异目的条带,阴性对照没有条带出现(图 1b).
-
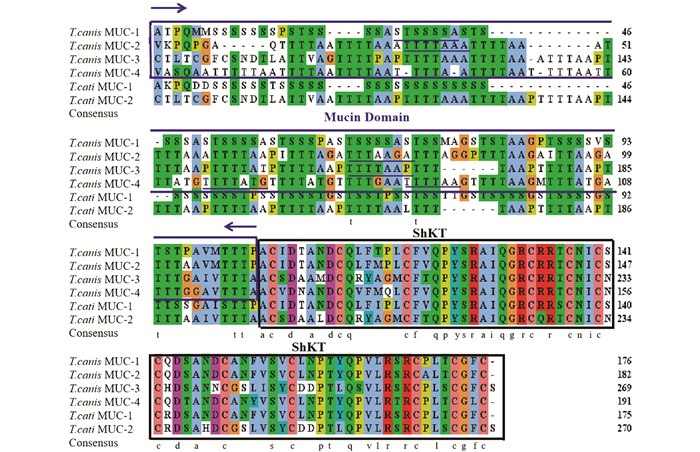
将测序获得的Tc-MUC-2氨基酸序列与犬/猫弓首蛔虫的其他黏蛋白Tc-MUC-1 (GenBank NO. AAB05820),Tc-MUC-3 (GenBank NO. AAD49340.1),Tc-MUC-4 (GenBank NO. AAD49341.1),T.cati-MUC-1 (GenBank NO. AZJ17292.1),T.cati-MUC-3 (GenBank NO. AZJ17291.1)氨基酸序列进行多重序列比对,结果显示犬弓首蛔虫的黏蛋白均含有高度保守的ShKT结构域,该结构域共有36个氨基酸,含有6个保守的半胱氨酸和许多其他保守的残基(图 2). 此外,犬弓首蛔虫黏蛋白还具有串联重复序列组成的黏蛋白结构域.
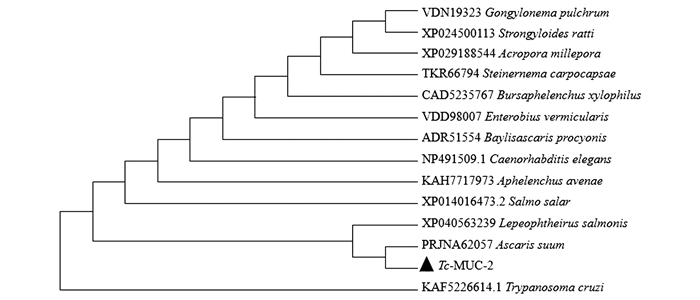
将Tc-muc-2编码的氨基酸序列与Warmbase Parasite和GenBank收录的贝氏蛔虫(Baylisascaris procyonis,GenBank NO. ADR51554)、松材线虫(Bursaphelenchus xylophilus,GenBank NO. CAD5235767)、小卷蛾斯氏线虫(Steinernema carpocapsae,GenBank NO. TKR66794)、筒线虫(Gongylonema pulchrum,GenBank NO. VDN19323)、蠕形住肠线虫(Enterobius vermicularis,GenBank NO. VDD98007)、燕麦滑刃线虫(Aphelenchus avenae,GenBank NO. KAH7717973)、鲑鱼海虱(Lepeophtheirus salmonis,GenBank NO. XP_040563239)、秀丽隐杆线虫(Caenorhabditis elegans,GenBank NO. NP_491509.1)、多孔鹿角珊瑚(Acropora millepora,GenBank NO. XP_029188544)、鼠类圆线虫(Strongyloides ratti,GenBank NO. XP_024500113)、日本血吸虫(Schistosoma japonicum,GenBank NO. PRJEA34885)、猪蛔虫(Ascaris suum,GenBank NO. PRJNA62057)、大西洋蛙(Salmo salar,GenBank NO. XP_014016473.2)氨基酸序列进行比对并构建进化树(图 3),结果显示Tc-MUC-2与猪蛔虫(A. suum,GenBank NO. PRJNA62057) 形成单独分支,进化关系较近.
-
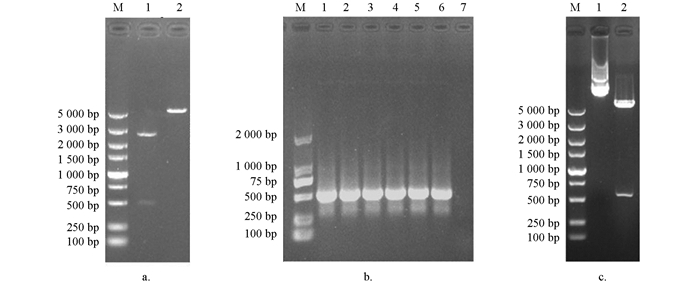
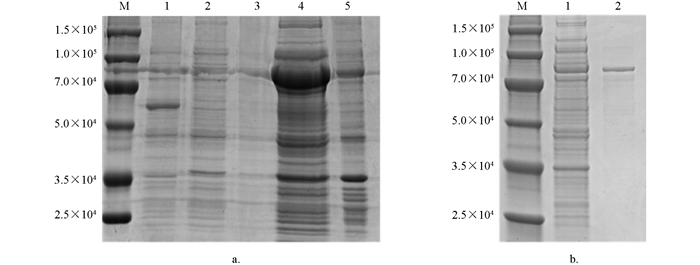
将测序成功的Tc-muc-2序列亚克隆至pCold TF表达载体,经菌液PCR检测,可见1%琼脂凝胶电泳结果具有清晰明亮的条带,且大小与预期相符(图 4b);经双酶切鉴定,结果表明已成功连接至pCold TF表达载体(图 4c).
-
将阳性重组质粒转化至E. coli BL21(DE3)感受态细胞后,经IPTG体外诱导表达,产物大部分以可溶性蛋白的形式存在,少量形成包涵体;重组蛋白Tc-MUC-2/pCold TF相对分子质量约为7.5×104;利用Ni-NTA对重组蛋白进行纯化,纯化后的蛋白经SDS-PAGE检测无杂带,具有较高的浓度和纯度(图 5).
-
将纯化后的重组蛋白Tc-MUC-2包被96孔板,利用间接ELISA法测定兔抗Tc-MUC-2多克隆抗体的效价,结果显示其抗体效价大于1∶512 000,可用于后续试验(表 1).
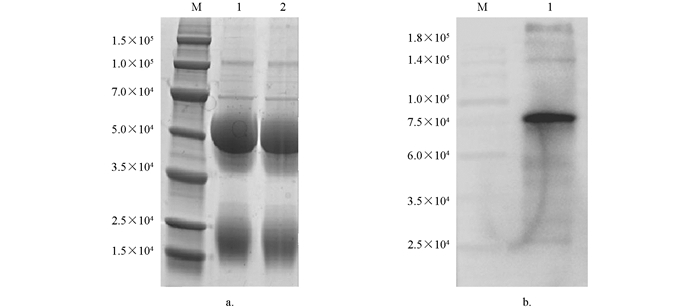
采用碧云天Protein A+G Agarose抗体纯化试剂盒对兔抗Tc-MUC-2多克隆抗体进行纯化,然后进行SDS-PAGE电泳检测,结果显示多克隆抗体含有重链和轻链,无明显杂带,纯度大于95%(图 6a);Western Blot检测结果显示兔抗Tc-MUC-2多克隆抗体能与Tc-MUC-2蛋白特异性结合,表明其特异性良好(图 6b).
2.1. PCR扩增及重组质粒鉴定结果
2.2. 多重序列比对及种系发育进化树
2.3. Tc-muc-2/pCold TF表达质粒的构建(图 4)
2.4. 重组蛋白的诱导表达及纯化
2.5. 多克隆抗体的纯化及鉴定
-
黏蛋白2(MUC-2)是一种高度糖基化的分泌型黏蛋白,由杯状细胞分泌,被包装成颗粒运输到细胞表面并最终释放入肠腔,作为肠黏液层的主要组成部分,其O-型糖基化程度决定黏蛋白对肠粘膜的保护作用[11-13]. Bergstrom等[14]发现MUC-2串联重复序列的密集糖基化使其能够作为保护屏障,在生物体和外部环境之间形成物理、化学和免疫学阻断. Nieuw等[15]发现MUC-2聚糖的硫酸化修饰可直接抑制细菌糖苷酶的活性,在宿主肠道内发挥保护作用.
本研究发现犬弓首蛔虫黏蛋白(Tc-MUC-2)具有黏蛋白结构域(Mucin domain)和ShKT结构域(ShKT domain). 多重序列比对显示Mucin domain由不同串联重复序列组成,如Tc-MUC-1含有11个串联重复的STSSSSA,Tc-MUC-2含有6个串联重复的TTTTAAA/TTTAAGA,Tc-MUC-3含有10个串联重复的TTTTAAP,Tc-MUC-4含有6个串联重复的TTTTAA/TTTTATG. ShKT结构域是来自海葵(Stichodactyla helianthus)的Ⅰ型钾通道毒素,Shafee等[16]证明ShKT结构可有效阻断电压门控钾(KV)1.3通道,该通道调节T细胞的膜电位,促进和维持Ca2+信号转导,在效应T细胞和记忆T细胞活化中起关键作用. Chhabra等[17]报道马来布鲁线虫(Brugia malayi)的ShK样结构域对大鼠和人类T细胞具有免疫调节功能.
原核表达系统常用载体有pET30a,pET28a,pET28b和pET32a等[18],诱导表达的产物主要以包涵体的形式存在,因此蛋白纯化时必须利用尿素进行变性处理,操作复杂且导致蛋白活性降低. pCold TF是一种冷休克表达载体,其低温诱导条件可控制蛋白表达的浓度,使蛋白有充分的空间进行折叠,且Trigger Factor(TF)能促进新生肽链的共翻译折叠,有效提高蛋白的溶解度[19-20]. 本试验成功构建了原核表达质粒Tc-muc-2/pCold TF,在OD600为0.8的菌液中加入IPTG,15 ℃诱导24 h获得大量可溶性的目的蛋白,其纯化条件为非变性条件,可以保证蛋白的完整性,并降低了试验中的操作难度.
多克隆抗体的评价主要有抗体效价和抗体特异性两个因素. 在抗体效价方面,本试验采用弗氏佐剂降低重组蛋白的释放速率,从而加大机体的吸收率,同时增强了Tc-MUC-2的免疫原性. 在抗体特异性方面,本试验对重组蛋白的纯化条件进行了优化,经过对比50 mmol/L,100 mmol/L,250 mmol/L和500 mmol/L不同浓度咪唑的洗脱效果,最终选择了250 mmol/L浓度咪唑进行洗脱,该条件获得的蛋白具有较高的浓度和纯度,进一步保证了多克隆抗体的特异性. 间接ELISA结果显示多克隆效价大于1∶512 000,Western Blot结果显示制备的多克隆抗体能特异性识别Tc-MUC-2. 本试验制备的重组蛋白及多克隆抗体,为后续研究Tc-MUC-2的生物学功能奠定了基础.






 DownLoad:
DownLoad: