-
开放科学(资源服务)标志码(OSID):

-
乳腺是哺乳动物周期性变化器官,不仅随动物年龄变化,还受到激素及相关信号因子的调控. 动物性成熟后经历妊娠期、泌乳期和退化期,对于哺育后代有着重要的作用[1]. STAT5是信号转导和转录激活子(STATs)家族中的一类蛋白,有研究表明其在细胞生长、代谢和泌乳等方面有着重要的影响[2]. Kang等[3]指出,STAT5可以影响乳腺上皮细胞的生长和泌乳功能,在乳腺上皮分化过程中,STAT5与H3K9me3结合,通过激活特异性基因表达在激素调控中发挥作用.
STAT5a和STAT5b 是STAT5的两个亚型,结构同源性虽然在90%以上,但是从不同的基因序列转录后,翻译得到的蛋白功能也不同. 当单敲除小鼠 STAT5a 基因时,产生的雌性小鼠后代在妊娠期间乳腺小泡生长被抑制,终末分化失败,导致分娩后乳腺无法正常泌乳[4]. 在乳腺上皮细胞中, STAT5a 对Janus激酶2(Jak2)介导的磷酸化、二聚化和易位到细胞核的催乳素泌乳过程作出反应[5-6]. STAT5a 与乳蛋白基因调控序列中的干扰素激活序列(GAS)元件结合,从而启动基因转录和乳蛋白合成. 通过单核苷酸多态性(SNP)分析,发现 STAT5a 有一个新的SNP(A/G),并分为AA/AG/GG基因型,不同基因型与奶牛的产奶量及奶品质息息相关[7]. 此外, STAT5a 还是奶牛健康泌乳的关键基因[8]. 因此,基于 STAT5a 在以上研究中的关键作用,深入地了解 STAT5a 的调控机制尤为重要.
花斑抑制因子同源物( SUV39H1/2 )是组蛋白甲基化转移酶,在哺乳动物染色质形成方面发挥重要作用[9]. SUV39H1/2 能够介导组蛋白3赖氨酸9三甲基化(H3K9me3)重编程障碍,导致染色质浓缩形成异染色质[10]. Chen等[11]发现 SUV39H1 减少与慢性阻塞性肺疾病(COPD)的异常炎症有关,COP39患者原代人小气道上皮细胞中过表达 SUV39H1 提高了H3K9me3水平并抑制了炎症,在COPD小鼠中,使用chaetocin抑制SUV39H1/H3K9me3水平时,炎症反应增强. Carvalho等[12]发现慢性淋巴细胞白血病(CLL)患者 SUV39H1 和 SUV39H2 的表达与染色体异常相关.
目前,尚不清楚 STAT5a 在水牛乳腺发育和泌乳中的作用及 SUV39H1/2 对其的调控机制. 为此,本研究首先克隆水牛 STAT5a 基因启动子,利用双荧光素酶法对启动子活性进行分析,然后采用RNA干扰技术抑制 SUV39H1/2 基因表达,探讨其对水牛乳腺上皮细胞增殖及 STAT5a 启动子甲基化程度的影响,以期进一步了解水牛产奶特性,并阐明 STAT5a 在水牛乳腺发育和泌乳方面的分子机理.
HTML
-
本试验所用水牛乳腺组织均来自广西本地屠宰场,采用组织块培养法得到原代水牛乳腺上皮细胞. 广西本地水牛 SUV39H1/H2 基因的shRNA序列采用林浪[13]、代小丽[14]的方法. pSico-GFP-SUV39H1-shRNA,pSico-GFP-SUV39H2-shRNA以及其他慢病毒包装质粒均为本实验室保存.
-
基因克隆引物(上海生工公司);兔多克隆H3K9me3抗体(CST公司);POLY-L-Lysine多聚赖氨酸,二抗TRITC goat-anti-rabbit IgG(武汉三鹰);细胞转染试剂购自全式金生物;细胞培养所用试剂及药品购自Sigma公司.
-
c-1000 Touch PCR仪、CKX41倒置显微镜(日本OLYMPUS);BioSpec-nano微量分光光度计(日本岛津);Gel DocTM XR+凝胶成像系统(美国BIO-RAD公司).
-
利用Oligo 6.0软件设计扩增上游启动子引物序列(表 1).
-
提取水牛卵巢组织的DNA,以水牛卵巢基因组DNA为模板扩增 STAT5a 启动子片段,然后进行PCR产物鉴定、胶回收、转化、重组质粒鉴定及质粒提取.
利用启动子结合位点分析软件TFSEARCH,Promoter SCAN,TSSW分析克隆得到序列启动子各个启动元件的位置及潜在的转录因子结合位点. 利用Meth Primer软件在线分析克隆得到序列的甲基化情况,在线预测 STAT5a 启动子区甲基化CpG岛.
-
将pMD-18T- STAT5a -0.3K,pMD-18T- STAT5a -0.4K,pMD-18T- STAT5a -2K和pGL3-Basic酶切纯化后,分别与pGL 3-Basic载体进行T4连接,然后进行重组质粒鉴定和去内毒素质粒提取. 转染至293T细胞进行启动子活性分析,荧光素检测依照Dual Luciferase Reporter Assay System (Promega)Ⅱ说明书进行.
-
通过双酶切来鉴定质粒大小的正确性后,参考林浪[13]的方法采用3质粒系统进行病毒包装,包括包膜质粒VSVG、包装质粒NRF和目的质粒pSicoR-GFP-SUV39H1-shRNA/pSicoR-GFP-SUV39H2-shRNA/pSicoR-GFP-control. 细胞增殖及周期检测采用CCK8试剂盒绘制细胞生长曲线,通过流式细胞仪检测细胞周期分布情况.
-
采用Trizol法提取乳腺上皮细胞(对照组和病毒感染组)总RNA,并用RT-PCR法反转录为cDNA. 以稀释后的cDNA为模板,加入特异性定量PCR引物(表 2). 反应体系:SYBR Premix EX TaqTM 10 μL、Rox 0.4 μL、上下游引物各0.3 μL、cDNA 1.0 μL、8.0 μL ddH2O. 反应程序为:95 ℃,5 min;95 ℃,30 s;60 ℃,30 s;72 ℃ 30 s,循环40次. 每个样品均重复3个反应,生物学重复3次. 以Histone为内参基因,采用2-ΔΔCT法计算mRNA相对定量值.
-
分别消化第5代未处理的水牛乳腺上皮细胞和慢病毒感染的乳腺上皮细胞,配置成1.5×103的细胞悬液,传入48孔板中每组3个重复. 细胞生长到80%时,换液,用PBS洗3遍. 加入4 mL/L多聚甲醛1 mL,4 ℃固定30 min. 吸去废液,用PBS清洗3次,每次3 min. 加入1 mL/L Triton X-100中透化20 min. 用PBS清洗3次,每次3 min. 每个孔细胞加入1 mL/L BSA封闭10 h. 弃废液,用PBS清洗3次,每次30 s,加入比值为1∶200的H3K9me3一抗使液面完全铺满细胞表面,孵育1 h. 回收一抗,用PBS清洗3次,每次3 min. 加入200 μL比值为1∶200的二抗中孵育45 min. 然后用DAPI染核15 min,PBS清洗3次. 封片后用荧光显微镜进行观察拍照.
-
试验结果采用SPSS Statistics V22.0软件进行分析. 每个试验重复至少3次,p<0.05表示差异具有统计学意义,p>0.05表示差异不具有统计学意义.
1.1. 材料
1.1.1. 样品采集
1.1.2. 主要试剂
1.1.3. 主要仪器及设备
1.2. 方法
1.2.1. 引物设计与合成
1.2.2. STAT5a 启动子克隆和生物信息学分析
1.2.3. STAT5a 启动子表达载体构建及活性分析
1.2.4. 慢病毒感染广西本地水牛乳腺上皮细胞
1.2.5. 荧光定量PCR
1.2.6. 免疫荧光法检测细胞H3K9me3水平
1.3. 数据分析
-
从加州大学旧金山分校(UCSC)数据库中导出牛 STAT5a 基因上游2 000 bp序列,利用Oligo 6.0软件设计两对特异性PCR引物,扩增得到 STAT5a 启动子约2 000 bp序列,克隆连接到pMD-18T载体,获得的阳性重组质粒命名为pMD-18T- STAT5a -2k. 将质粒送华大基因测序,结果显示序列同源性达99%.
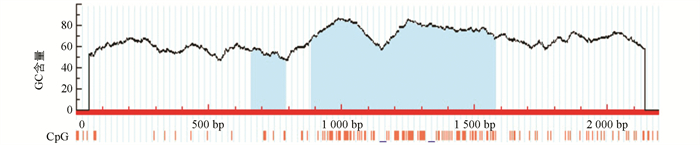
利用Methprimer在线分析 STAT5a 启动子区CpG岛情况. 分析标准为Window>100 bp,GC%>50%,Obs/Exp>0.6. 结果显示, STAT5a 启动子序列存在2个CpG岛(图 1). 利用启动子在线分析软件TFSEARCH,Promoter SCAN,TSSW,推测克隆得到的2 190 bp片段存在两个启动子区,且转录因子以 Sp1,p53,AP1 为主(表 3).
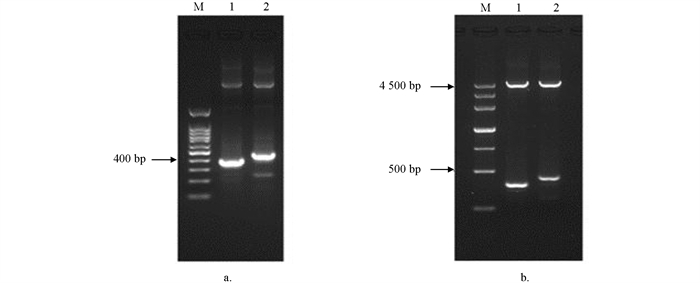
根据在线软件分析获得 STAT5a 启动子活性区为754~1 004 bp和1 025~1 275 bp,通过Oligo 6.0软件设计两对特异性PCR引物. 以获得的 STAT5a 前2 000 bp序列为模板,扩增得到 STAT5a 前体上游两个特异性片段. 电泳条带与预期一致(图 2). 将PCR产物纯化后,分别与pMD-18T载体连接,筛选得到阳性重组质粒,命名为pMD-18T- STAT5a -0.3K和pMD-18T- STAT5a -0.4K. 经酶切鉴定后送华大基因测序获得355 bp和417 bp两段序列.
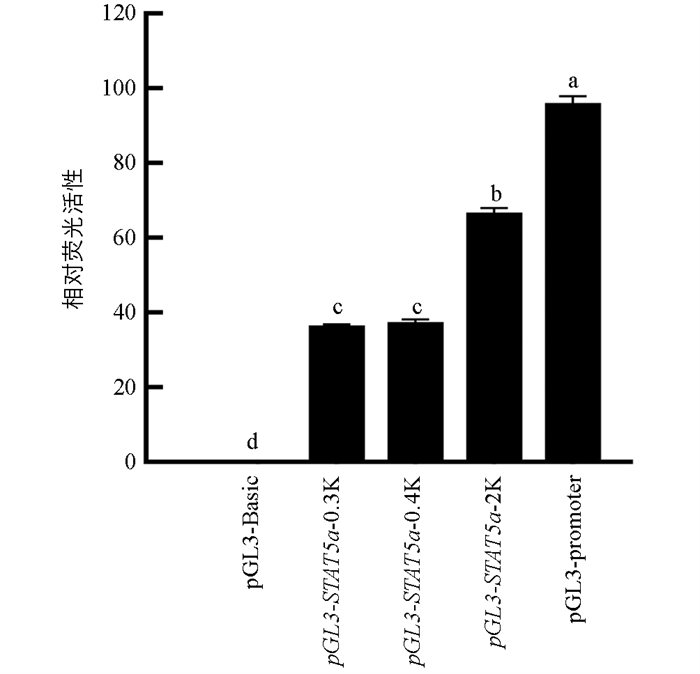
通过Promega公司Dual Luciferase Reporter Assay SystemⅡ报告系统检查荧光素酶的表达水平. 结果显示,与阴性对照组相比pGL3- STAT5a -0.3K,pGL3- STAT5a -0.4K和pGL3-promoter组表达均显著上调(p<0.05),pGL3- STAT5a -0.3K与pGL3-18T- STAT5a -0.4K组相比表达差异不明显(p>0.05),而pGL3-148a-2K和pGL3-promoter组表达显著高于pGL3- STAT5a -0.3K组(p<0.05),说明355 bp、417 bp和2 190 bp均具有启动子活性,2 190 bp活性显著高于其他两条片段(图 3).
-
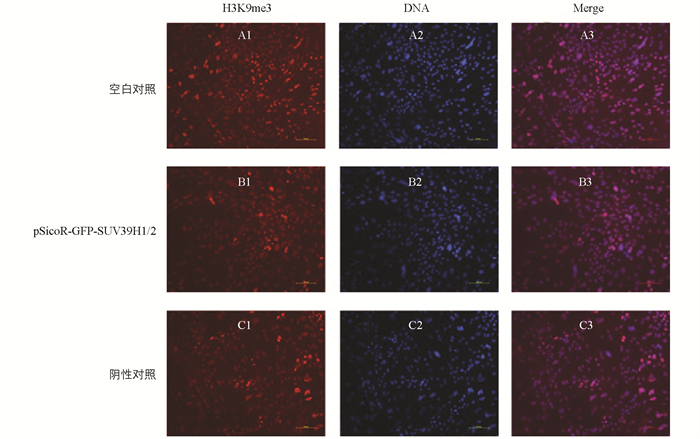
免疫荧光结果显示,与空白对照组和阴性对照组相比,实验组的水牛乳腺上皮细胞中,组蛋白H3K9me3水平显著降低(p<0.05)(图 4).
-
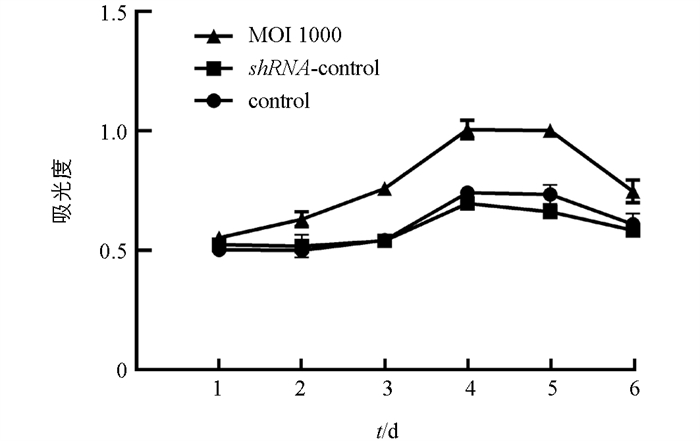
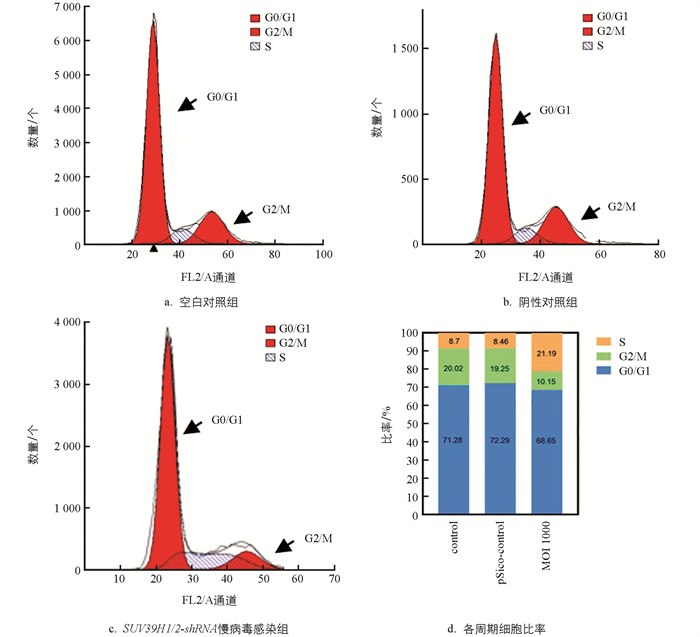
本研究发现,慢病毒感染抑制 SUV39H1/2 基因表达可促进乳腺上皮细胞增殖,与空白对照组和阴性对照组相比,实验组(MOI 1000)有显著差异(p<0.05)(图 5). 流式细胞仪检测发现,与空白对照组和阴性对照组相比,实验组细胞周期阻滞在S期,G0/G1期细胞比例无显著差异(p>0.05),G2/M期比例显著减少(p<0.05)(图 6).
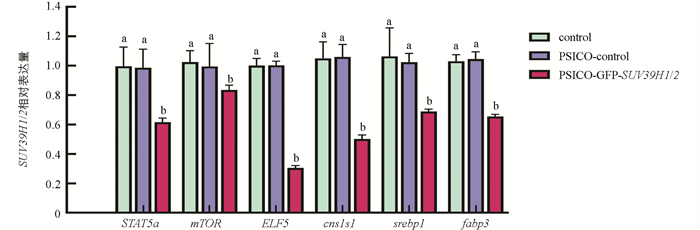
qRT-PCR结果显示,与空白对照组和阴性对照组相比,实验组乳腺上皮细胞分化基因 STAT5a ,mTOR表达显著降低(p<0.05);乳蛋白合成相关基因 ELF5 , Csn1s1 表达极显著降低(p<0.01); ELF5 , csn1s1 及乳脂合成相关基因 srebp1 , fabp3 的表达极显著降低(p<0.01)(图 7).
-
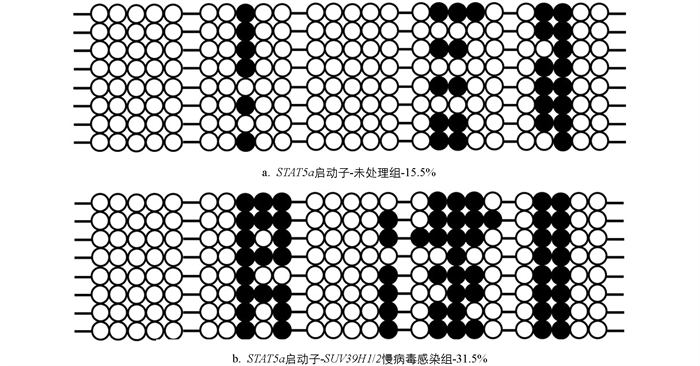
STAT5a 启动子区甲基化情况如图 8所示,未处理组 STAT5a 启动子区甲基化程度为15.5%,抑制 SUV39H1/2 表达组 STAT5a 启动子区甲基化程度为31.5%,表明 SUV39H1/2 能够通过影响 STAT5a 启动子区甲基化,从而影响 STAT5a 基因的表达.
2.1. STAT5a 启动子克隆及生物信息学分析
2.2. SUV39H1/2 基因表达及对组蛋白H3K9me3水平的影响分析
2.3. SUV39H1/2 基因表达下调对水牛乳腺上皮细胞增殖及泌乳相关基因表达的影响
2.4. SUV39H1/2 基因表达下调对 STAT5a 启动子区甲基化水平的影响
-
对哺乳动物乳腺 STAT5a 的研究发现,其转录起始位点的上游-679~0 bp处存在一个CpG岛,DNA甲基化可能是通过这些位点的甲基化水平来调控表达,其中包括 P300 和 SP1 转录因子的多个结合位点[15-16]. 吴贤锋[17]在对山羊的研究中发现, STAT5a 基因内含子CpG岛的甲基化状态影响了山羊的体质量、产奶量和乳密度. 本试验通过克隆的方法得到了水牛 STAT5a 基因前2 190 bp启动子区序列,通过序列比对,发现与黄牛 STAT5a 基因启动子区的相似度达97%. 采用生物信息学分析序列,发现其含2个CpG岛,存在多个高甲基化位点. 另外,在 STAT5a 启动子区,本研究还发现了多个转录因子结合位点 SP1,p53,p300 等,表明 STAT5a 受多种基因调控. 为了找到 STAT5a 核心启动子区,采用软件分析得到 STAT5a 启动子区无典型的启动子元件,根据预测评分较高的两个启动子区域克隆获得355 bp和417 bp片段,构建双荧光素酶载体,验证启动子活性,结果发现355 bp,417 bp,2 190 bp片段均具有启动子活性,且2 190 bp活性最高(p<0.05).
组蛋白H3K9me3与表观遗传转录抑制有关. 很多研究发现, SUV39H1/2 基因协同调控癌细胞H3K9me3在体内的动态变化来发挥其生物调节功能. 通过雷公藤甲素对前列腺癌(PCa)细胞的甲基化影响,人们发现其通过下调 JMJD3和UTX 来增强H3K27me3水平,同时还通过上调 SUV39H1 来增强H3K9me3,促进异染色质形成和在 E2F1 靶基因启动子上的沉积,降低细胞活力,诱导细胞衰老表型[18]. 有研究报道了SUV39H1通过与Rb协同作用于 E2F ,使细胞周期在G0/G1期和S期转换[19]. 本研究中抑制 SUV39H1/2 基因表达,同样看到细胞周期阻滞在S期,且G2/M期比例减少.
SUV39H1 与Rb1结合并通过H3K9me3对细胞周期蛋白基因启动子区域进行DNA甲基化富集,从而使细胞周期蛋白基因抑制. 与之相似的研究发现, SUV39H1 缺失的成纤维细胞中细胞周期蛋白E(CCNE1)和细胞周期蛋白A2(CyclinA2)基因的活性升高[20]. 起源识别复合体(ORC)的大亚基ORC1与SUV39H1和Rb蛋白协同抑制 CCNE1 转录,从而抑制组蛋白H3K9me3标记. CCNE1 延迟表达使新一代细胞可以选择增殖或退出细胞周期[21]. 为了研究 SUV39H1 诱导的异染色质调节是否会影响哺乳动物的发育,在小鼠胚胎发生早期过表达 SUV39H1 ,转基因小鼠生长发育迟缓,骨骼转化能力弱及红细胞分化受损. 在离体培养的成红细胞表现出异常的细胞周期特征,表现为较低的G0/G1期和较高的S期[22]. 本研究采用广西本地水牛乳腺上皮细胞,运用干扰RNA慢病毒感染技术抑制 SUV39H1/2 基因表达,使 SUV39H1和SUV39H2 基因表达均显著下调(p<0.05),组蛋白H3K9me3的表达也显著下调(p<0.05),并且促进细胞增殖,使细胞周期被阻滞在S期,G0/G1期细胞比例无显著差异,G2/M期比例显著减少(p<0.05).
定量PCR结果显示,与对照组相比,实验组中乳腺上皮细胞生长分化相关基因、乳蛋白合成相关基因、乳脂合成相关基因均显著下调(p<0.05). 其中,乳蛋白合成相关基因 ELF5 及功能性酪蛋白基因 Csn1s1 表达极显著下调(p<0.01),本研究推测可能与乳腺上皮细胞的细胞特异性有关. 与对照组(15.5%)相比,实验组(31.5%) STAT5a 启动子区甲基化程度显著升高(p<0.05),表明组蛋白H3K9me3下调对水牛乳腺上皮细胞 STAT5a 基因的作用,可能是通过改变 STAT5a 基因启动子区的DNA甲基化修饰,从而影响 STAT5a 基因的表达.
-
抑制 SUV39H1/2 基因表达能够促进水牛乳腺上皮细胞增殖,并显著下调 STAT5a ,mTOR, ELF5 , Csn1s1 , abp3 , srebp1 等与乳腺生长发育及泌乳相关基因的表达.






 DownLoad:
DownLoad: