-
开放科学(资源服务)标志码(OSID):

-
昆虫致病真菌附着于昆虫体表及体内,迅速增殖并致寄主死亡[1]. 真菌孢子附着并侵入昆虫体壁后在体腔内繁殖,菌丝利用宿主组织营养不断生长,最终导致虫体死亡. 据不完全统计,全球已记载的昆虫病原真菌约100属800多种[2]. 我国目前已经报道的昆虫致病真菌约400多种,其中寄生性昆虫致病真菌约200多种[3],主要经昆虫体壁入侵到宿主体内,有时也可以经呼吸道和消化道感染[4-5]. 在昆虫病原真菌入侵寄主时,寄主也会通过自身的体液免疫和细胞免疫产生应激反应,对真菌入侵具有一定的防御能力. 在自然界中由真菌引起的流行病可自然制约昆虫的种群数量,因此利用昆虫病原真菌进行生物防治已受到人们的青睐和重视.
拟青霉属Simplicillium是21世纪初由生命科学家划分出的一个新属,是一类寄生性真菌,属于肉座菌目Hypocreales虫草科Cordycipitaceae. Simplicillium属的分生孢子具球形粘性末端,透明,呈椭圆形、纺锤形或圆柱形等形态[6-8]. Simplicillium属真菌广泛分布于自然生态系统中,通常可寄生于植物组织、土壤、线虫及真菌蘑菇中[9-10],在生物防控和生物活性化合物应用上具有非常重要的作用,因此该菌具有较高的生态价值和经济价值.
为了解土壤真菌的多样性和分布情况,本试验采用平板稀释法,利用选择性培养基从土壤中分离并鉴定白色拟青霉S. lanosoniveum菌株;同时采用孢子浸渍法[11]筛选出对家蚕3龄幼虫具有高毒力的优势菌株,以期为白色拟青霉S. lanosoniveum菌株的生物防治奠定基础.
HTML
-
本试验分别从重庆市荣昌区普通土壤、玉米地土壤、香蕉地土壤、柑橘树土壤和李子树土壤这5种土壤类型中采集了6个土壤样本(表 1). 采集样本时,需先铲去表层约5 cm土壤,采集5~20 cm深度的土壤,过滤除去沙石,装进塑料袋中做好标签,4 ℃条件保存备用. 球孢白僵菌Beauveria bassiana购自广州多宇多生物科技有限公司. 金龟子绿僵菌Metarhizium anisopliae购自重庆聚立信生物工程有限公司. 家蚕Bombyx mori由家蚕基因组生物学国家重点实验室(西南大学)提供,饲养于16 cm×10 cm×8 cm透气打孔饲养盒中,需每天更换新鲜桑叶饲养.
PCR试剂(MgCl2、Buffer、dNTPs等)、rTaq酶、pMDTM19-T Vector、DL 2000 DNA Marker、琼脂糖、Amp、X-gal、IPTG购自大连宝生物公司;蛋白胨Tryptone、酵母提取物Yeast extract购自Oxiod公司;蛋白酶K购自Merk公司;DNA凝胶回收试剂盒、DH5α感受态细胞购自TransGen Biotech公司;重组质粒WizardTM Plus SV Minipreps DNA Purification System试剂盒购自Promega公司.
-
称取5 g土壤样本,加入灭菌蒸馏水45 mL,于漩涡振荡器振荡5 min,使震荡液散布均匀后稀释10倍. 移液枪吸取0.5 mL振荡液涂布于马铃薯蛋白胨培养基(PPDA),放置在27 ℃恒温保湿箱内培养,待平板生长出单菌落后挑取转至马铃薯培养基(PDA),需至少挑取3次以上PDA培养基单菌落才可纯化目的菌株.
-
将分离纯化的菌落接种于PDA培养基中央,27 ℃恒温保湿箱内培养,定期观察菌落生长状态并记录其颜色、形态,参考文献[12]对菌株进行形态学初步鉴定.
-
(1) 菌株DNA提取
刮取适量真菌分生孢子或菌丝于裂解管内,加入270 μL的SDS裂解液[13]和30 μL(50 μg/μL)蛋白酶K充分混匀,隔夜55 ℃水浴12~24 h,其间多次振荡混匀离心管使其裂解充分,采用酚/氯仿法抽提样本DNA. 提取出的DNA样品分装后,于-20 ℃冰箱保存备用.
(2) PCR扩增
参考Chen等[14]的真菌ITS(Internal Transcribed Spacer,ITS)引物扩增序列,该序列段位于18S rRNA和28S rRNA的保守区,核苷酸序列为:ITS-F:5'-TAACAAGGTCTCCGTTGGTGA-3' (21 bp);ITS-R:5'-TCCTCCGCTTATTGATATGC-3' (20 bp);引物由重庆擎科兴业生物技术有限公司合成. 使用时加灭菌超纯水稀释,稀释度为10 μmol/μL,分装后于-20 ℃保存备用. PCR反应体系为25 μL,其中3 μL Mg2+(25 mmol/L),2.0 μL dNTPs(2.5 μmol/L),2.5 μL 10×PCR buffer(不含Mg2+),0.5 μL上游引物ITS-F(10 μmol/L),0.5 μL下游引物ITS-R(10 μmol/L),2.0 μL模板DNA,0.2 μL rTaq酶(5 U/μL),灭菌双蒸水14.3 μL. 反应在PCR仪BIO RAD S1000上进行,预期扩增片段约为600 bp. 反应参数为预变性94 ℃ 3 min,变性94 ℃ 1 min,退火温度56 ℃ 1 min,延伸72 ℃ 1 min,循环反应35次,最后72 ℃延伸10 min,同时设置阴性对照组. PCR产物经10 g/L TAE琼脂糖凝胶电泳后,成像系统显示记录分析结果.
(3) PCR产物的克隆
按照北京全式金生物公司的Agrose Gel DNA Extraction Kit说明书进行凝胶回收. 将回收的PCR产物与pMDTM19-T Vector载体连接:取PCR管依次加入5 μL Ligation Solution I,1 μL pMDTM19-T Vector载体,3 μL PCR纯化产物,1 μL ddH2O,混匀,16 ℃ PCR扩增仪连接1 h. 连接后将上述连接产物转化至DH5α感受态细胞,涂布于含Amp、X-gal和IPTG的LB琼脂平板中,置恒温箱37 ℃过夜培养12~14 h后,筛选阳性克隆.
(4) 测序与序列分析
挑取阳性克隆菌液送重庆擎科兴业生物技术有限公司测序. 将测序结果于美国国家生物信息中心(NCBI)数据库进行BLAST比对,同时基于ITS下载了不同的序列:S. lanosoniveum(GeneBank NO. KT878334、MT001191),S. cylindrosporum(GeneBank NO. NR_111023),S. obclavatum(GeneBank NO. NR_111099),S. lamellicola(GeneBank NO. NR_111098),S. chinense(GeneBank NO. NR_155782),S. minatense(GeneBank NO. NR_111025),S. subtropicum(GeneBank NO. NR_111024),S. aogashimaense(GeneBank NO. NR_111026)和S. sympodiophorum(GeneBank NO. NR_111027);Lecanicillium kalimantanense(GeneBank NO. NR_121200),L. araneicola(GeneBank NO. NR_121208),L. primulinum(GeneBank NO. NR_119418)和枝顶孢属Acremonium sp.(GeneBank NO. EF577237). 采用MegAlign 5.0的邻接法构建种系发育进化树,进化树的可靠性用Bootstrap进行分析,共1 000个重复.
-
(1) 孢子悬液制备
菌株分生孢子在PDA培养基中长满,加入灭菌的0.05%吐温-80,将分生孢子连同孢子梗一同刮下,置于锥形瓶中,振荡器振荡30 min使分生孢子和菌丝分离,3层纱布过滤获得孢子悬液. 用血球计数板将孢子含量调成1×108个孢子/mL.
(2) 优势毒力菌株筛选
采用孢子液浸渍法[11]对家蚕3龄幼虫毒力进行测定. 挑选大小相近的健康试虫,将其浸入孢子悬液中约6 s,取出用滤纸吸干多余水分后,放回培养盒,饲养温度为(25±1) ℃,相对湿度为90%,每天及时饲养新鲜桑叶并清理粪便颗粒,发现病死虫体即挑出,保持饲养环境干净. 每天记录家蚕3龄幼虫的死亡情况,连续观察12 d. 每处理3个重复,每盒30头. 阴性对照组为0.05%吐温-80无菌水代替孢子悬浮液浸虫,筛选出高致病力菌株. 阳性对照组为球孢白僵菌B. bassiana和金龟子绿僵菌M. anisopliae,以及阴性对照组(CK)所需的0.05%吐温-80无菌水,以评估S. lanosoniveum的致病效果.
(3) 数据分析
数据分析应用Excel(2010)先计算出接种后家蚕的死亡率及僵虫率,用DPS(V 9.01)软件处理试验数据,求出毒力回归方程,计算其致死中时间LT50(median lethal time),据此分析菌株的毒力[15]. 死亡率和校正死亡率的计算采用Abbott公式校正[16].
公式为:
式中:A代表死亡率,a代表死亡虫数,Z代表供试虫数,B代表校正死亡率,b代表处理死亡率,c代表对照死亡率.
1.1. 材料
1.2. 方法
1.2.1. 菌株的分离纯化
1.2.2. 菌株的形态学鉴定
1.2.3. 菌株的分子生物学鉴定
1.2.4. S. lanosoniveum优势毒力菌株筛选
-
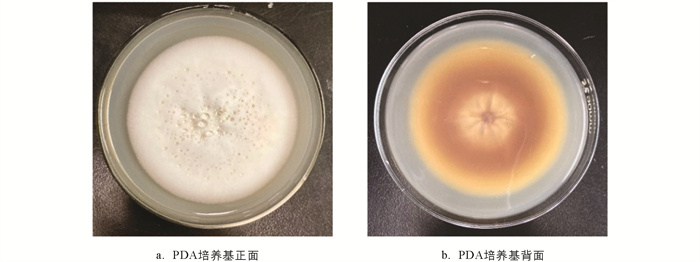
菌株分离纯化培养2周后,菌落正面呈白色,直径约8 cm,圆形,有同心环纹,发达的绒毛状菌丝,奶油絮状或棉花样,质地粗厚干燥,结构致密,不透明;发育成熟过程中背面菌落由白色变为淡黄色,呈放射状裂纹(图 1). 根据上述形态学观察结果,初步判定分离所得的6株土壤分离株为虫草科Cordycipitaceae,Simplicillium属真菌. 将本次试验的土壤分离株分别编号为S. lanosoniveum-Sl01,Sl02,Sl03,Sl04,Sl05,Sl06.
-
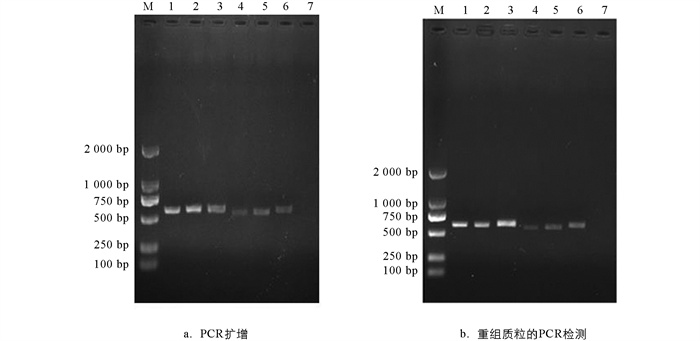
为进一步确定样本菌株的种属地位,本文基于ITS序列进行了分子鉴定. 结果显示,PCR扩增产物经1%琼脂糖凝胶电泳后,6株菌株均扩增出约616 bp的条带(图 2a);阳性克隆的重组质粒经菌液PCR同样扩增出大约616 bp的ITS序列条带(图 2b). 扩增条带与预期片段大小相符,阴性对照没有出现条带.
-
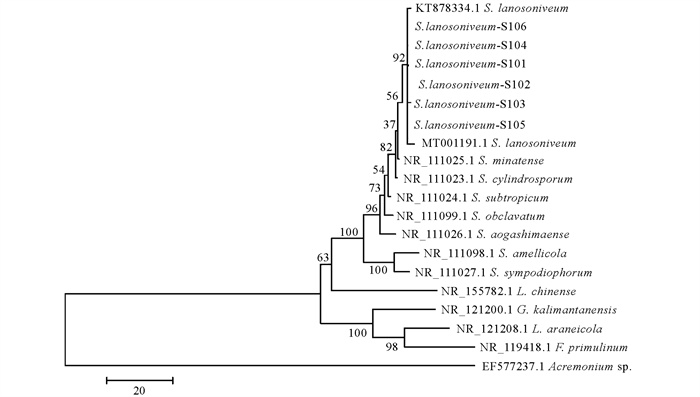
对PCR鉴定为阳性的重组菌液进行测序. 结果显示,6株菌株获得的PCR产物序列长度一致,均为616 bp. 经NCBI网站BLAST比对,本研究获得的真菌ITS序列与S. lanosoniveum (GeneBank NO. KT878334、MT001191)的同源性达99.9%~100%;对本次研究所得的6株S. lanosoniveum序列与下载的14株序列应用程序软件MegAlign 5.0中的Neighbour-Joining构建种系发育进化树(图 3),结果显示6株S. lanosoniveum与GeneBank登记的S. lanosoniveum (GeneBank NO. KT878334、MT001191)位于同一个分支上,表明其遗传距离最近;与Acremonium sp. (GeneBank NO. EF577237)的遗传距离最远.
-
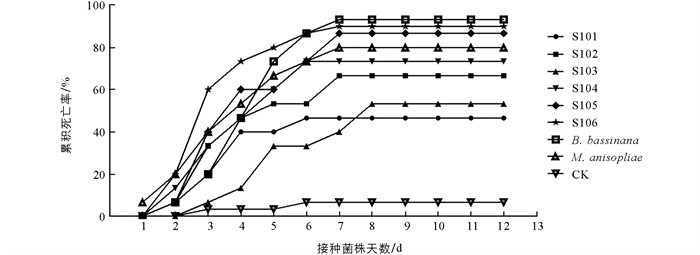
家蚕3龄幼虫接种不同菌株后累积死亡率见图 4. 不同菌株对家蚕3龄幼虫均具有致病性,累积死亡率随被侵染时间延长而增大. 累积校正死亡率可以反映出毒株致病力的强弱,累积校正死亡率越高,致病力越强. 球孢白僵菌对照组累积校正死亡率最高,达92.30%;接种分离菌株S. lanosoniveum-Sl05,Sl06后第8 d累积校正死亡率大于80.00%,而其余4株S. lanosoniveum-Sl01,Sl02,Sl03,Sl04累积校正死亡率分别为30.78%,61.54%,46.15%,69.23%.
不同菌株对家蚕3龄幼虫的毒力回归方程如表 2所示. 其中,S. lanosoniveum-Sl05,Sl06致死中时LT50分别为3.96 d和3.73 d,小于其他同期S. lanosoniveum菌株处理组. 虽然S. lanosoniveum-Sl04致死中时LT50为4.11 d,同样具有优势,但是累积校正死亡率为69.23%,相对较低. 阳性对照组球孢白僵菌和金龟子绿僵菌LT50分别为3.92 d和3.63 d.
综合校正死亡率和致死中时两个指标,在6株样本菌株中S. lanosoniveum-Sl06的校正死亡率最高,为89.29%,致死中时(LT50)最短,为3.73 d. S. lanosonievum-Sl05次之,校正死亡率为84.62%,致死中时(LT50)为3.96 d. 这两株菌株表现出良好的毒力活性,是S. lanosoniveum家蚕3龄幼虫致病的优势菌株.
2.1. 菌株的形态学鉴定
2.2. 菌株的分子生物学鉴定
2.3. 种系发育进化树分析
2.4. S. lanosoniveum优势毒力菌株的筛选
-
土壤中昆虫致病真菌种类繁多,其中最大生防类群的虫生真菌占比达60%以上,表明土壤能为虫生真菌研究提供最基础且最丰富的来源[17]. Simplicillium属真菌在土壤、海水、空气等环境中的生活模式丰富多样[14],可作为共生真菌、内生真菌、昆虫致病真菌和真菌寄生菌被分离出来. 土壤中虫生真菌菌株的分离方法通常有两种,即僵虫分离法和土壤分离法[18]. 其中,土壤分离法更加简便易行、环保有效,故常被研究人员使用. 王品[19]利用选择性培养基标准平板技术,对我国4大茶区茶园土壤中的虫生真菌进行分离,成功分离出4属11种虫生真菌菌株. 本试验同样采用土壤分离法成功分离出了6株S. lanosonievum菌株,表明土壤中存在一定数量的昆虫致病真菌,且使用土壤分离法确实简便易行. 据耿敬可等[20]的研究报道,虫生真菌的种属特异性较强,其宿主谱和毒力会因菌株不同而呈现出差异. 因此,对土壤中更多未知菌种进行分类鉴定,将有利于丰富和增加虫生真菌资源库的资源.
真菌种类的鉴定包括形态学鉴定和分子生物学鉴定. 传统的形态学鉴定主要是通过对培养后真菌的菌落形态和显微镜下分生孢子、菌丝等的生长特征来进行鉴别[15]. 由于生长的营养条件或者环境不同,真菌生长形态会发生改变,并且自然界中真菌种类繁多,存在许多外观相似的物种,若只进行形态学鉴定很难对其进行准确的属种分类,而分子生物学技术则能很好地解决近缘种之间分类难的问题. 分子鉴定中常用18S rDNA,ITS和线粒体序列[21]等对真菌进行鉴定. 其中,核糖体r DNA中内转录间隔区ITS在真菌种内高度保守,而在种间具有显著而丰富的变化[22],在真菌物种亲缘关系的研究中具有很高的参考价值,因此被广泛应用于真菌鉴定及系统发育研究[23]. Chen等[14]从中国西南地区分离纯化出3种新的Simplicillium属菌株,命名为S. cicadellidae,S. formicidae和S. lepidopterorum. 经形态学观察、分子鉴定及系统发育分析,最终确定为3个新物种,从而丰富了中国西南地区的虫生真菌资源. Baiswar等[24]从印度阔叶树患病叶片中分离出一株昆虫致病真菌,经扫描电镜下形态表征、ITS序列分析及系统发育分析,也鉴定为S. lanosonievum. 本试验从不同土壤中获得6株真菌,经ITS克隆测序,结果长度均为616 bp,表明ITS序列具有较高的保守性. 系统发育进化树分析显示,6株真菌与已登录的S. lanosoniveum(GeneBank NO. KT878334、MT001191)位于同一分支,相似性为99.9%~100%,与S. minatense亲缘关系最近,而与形态相似的枝顶孢属Acremonium sp.亲缘关系最远,表明ITS序列种内差异较小,种间差异大,可作为S. lanosoniveum菌株鉴定的遗传标记.
昆虫病原真菌是昆虫病原微生物中最大的类群,所产生的流行病对自然界中害虫种群数量的控制具有关键性作用. 因此,寻找高毒力的虫生真菌菌株,开发以其为主要成分的真菌杀虫剂具有广阔的应用前景. Sujithra等[25]从褶皱螺旋粉虱若虫尸体中分离出一株昆虫致病真菌,鉴定为S. lanosonievum;以浸渍接种法对粉虱发育的各个时期进行毒力测试,发现其对粉虱虫卵、1~3龄若虫和蛹等阶段均具有较高的毒力. 孙明等[26]从土壤中分离的球孢白僵菌,筛选出了对多种蜱致死率达100%的高毒力菌株. 本试验获得的6株S. lanosoniveum毒力有所差异,其中S. lanosonievum-Sl05,Sl06对家蚕3龄幼虫的累积校正死亡率大于80.00%,最高可达89.29%,具备较高的毒力,说明本研究从土壤中分离筛选的昆虫病原真菌具有生物防治的应用前景.
-
从土壤中分离的6株真菌,经形态学和分子生物学鉴定为S. lanosoniveu真菌,其中S. lanosoniveum-Sl06和S. lanosonievum-Sl05菌株感染家蚕3龄幼蚕,两株菌株均表现出良好的毒力活性,是S. lanosoniveum致病家蚕3龄幼虫的优势菌株.






 DownLoad:
DownLoad: