-
开放科学(资源服务)标志码(OSID):

-
甘薯[Ipomoea batatas (L.) Lam.]种植于世界上热带和亚热带地区的100多个国家,是世界上最重要的粮食作物之一[1]. 已有研究表明甘薯茎叶中富含蛋白质、多酚、黄酮、花青素、多糖和一些多功能营养素[2-3],有益于人体健康,如抗癌、保肝、抑菌、抗艾滋病病毒和提高人体免疫力、预防糖尿病和心血管病等[4-5]. 甘薯种植主要收获对象是块根,只有很少部分茎叶用于牲畜饲用和人类食用,绝大多数被丢弃,造成了极大的资源浪费[6].
酚类物质是广泛存在于植物中的膳食抗氧化剂,甘薯叶中多酚化合物主要是绿原酸及其衍生物[7]. 绿原酸(Chlorogenic acid,CGA)是由咖啡酸(Caffeic acid)与奎宁酸(Quinicacid)生成的缩酚酸,是植物体内有氧呼吸过程中经莽草酸途径产生的一种苯丙素类次生代谢产物,具有多种生物活性[8-9],如抗氧化作用、清除自由基、抑菌作用、抗病毒作用、调节糖脂代谢、免疫调节及抗肿瘤作用等[10-14]. 在甘薯叶中至少包括6种咖啡酰奎宁酸衍生物,分别为单咖啡酰奎宁酸(CQA)的3种异构体:绿原酸(3-CQA)、隐绿原酸(4-CQA)和新绿原酸(5-CQA),以及3种二咖啡酰奎宁酸:异绿原酸A(3,5-diCQA)、异绿原酸B(3,4-diCQA)和异绿原酸C(4,5-diCQA). 甘薯CGA质量分数主要取决于遗传特性,除此之外,生育期对甘薯叶片中酚酸质量分数也有较大的影响,同一基因型叶片中的CGA质量分数远高于块根[15-17].
甘薯茎叶提取物具有较强的抗氧化能力,其抗氧化能力与茎叶中的CGA、黄芪素和矢车菊素类化合物相关,其中与CGA及其衍生物的相关性最高[16, 18-19]. 如Xu等[20]通过对蒲薯53叶提取物进行成分与抗氧化活性分析,表明提取物抗氧化活性的主要生物活性化合物是多酚类,尤其是CQA衍生物.
目前对于甘薯CGA的研究主要存在供试材料偏少的问题,且大多研究薯块,对于较多基因型间CGA组分分析更为少见. 因此,本研究通过高效液相色谱法(High Performance Liquid Chromatography,HPLC)对50个甘薯基因型叶片中CGA组分的质量分数进行测定并对其抗氧化活性进行分析,以期为甘薯高绿原酸、高抗氧化活性种质资源的筛选及功能茎叶品种的选育、甘薯茎叶营养功能食品的开发利用奠定基础.
HTML
-
供试的50个甘薯基因型来自西南大学重庆市甘薯工程技术研究中心合川农场育种基地的育成品种或育成品系比较实验. 供试基因型的编号、名称见表 1.
-
供试材料大田移栽100 d时,取甘薯藤蔓自上而下第3~10片新鲜无病斑、无腐烂的健康叶片,用流水轻轻洗净泥沙杂质后,自然晾干多余水分,叶片于105 ℃杀青后,60 ℃烘干至恒质量,粉碎,过100目筛,-20 ℃密封保存备用.
参照Zhang等[21]描述的方法,略作修改. 精确称取甘薯叶粉末0.05 g,按料液比1∶100(g/mL)与70%乙醇混合,于60 ℃下超声提取1 h,提取2次,每次的提取液4 000 r/min离心40 min,合并上清液,经0.22 μm醋酸纤维膜过滤,滤液用于CGA质量分数与组分测定及抗氧化能力研究.
-
Agilent色谱柱(型号TC-C18,250 mm×4.6 mm,5 μm),0.2%的甲酸(A)-乙腈(B)为流动相,线性梯度洗脱:0~5 min为95%~90%B,5~45 min为90%~55%B,45~50 min为95%B;流速1 mL/min,柱温40 ℃,进样体积10 μL,紫外检测波长327 nm.
-
3-CQA,4-CQA,5-CQA,3,5-diCQA,3,4-diCQA,4,5-diCQA标准品购自美国Sigma-Aldrich公司,分别称定1.000 mg,以70%乙醇溶解定容后进行系列稀释,分别配成5,10,20,40,60,80,100 μg/mL的系列混合标准品溶液.
-
分别取混合标准品溶液和供试提取液,按照1.2.2.1色谱条件各进样10 μL,记录色谱图,分析各峰分离度,记录峰面积和保留时间,计算相对标准偏差(Relative standard deviation,RSD)值以进行系统适用性实验、精密度实验、稳定性实验. 以浓度为横坐标,峰面积为纵坐标,进行线性关系考察.
-
将样品提取液进样10 μL,按照1.2.2.1色谱条件进行进样分析,得到峰面积,计算各组分的质量分数.
-
ABTS自由基清除实验参考Re等[22]描述的方法,将7 mmoL/L ABTS+溶液与2.45 mmoL/L的K2S2O8溶液1∶1混合过夜反应得到ABTS+储备液. 将储备液稀释至在734 nm处达到0.7±0.02的吸光度以得到ABTS+工作液. 取0.4 mL提取液与3.6 mL ABTS+溶液混合,室温反应20 min,于734 nm波长下在酶标仪上测定吸光值,并根据水溶性维生素E(Trolox)制成的标准曲线(Y=0.226 4X+0.531 6,R2=0.999 1)计算清除能力.
-
DPPH自由基清除能力实验参照Yamaguchi等[23]实验方法,取提取液2 mL与2 mL 0.2 mmoL/L的DPPH溶液混合,室温避光反应30 min,于517 nm波长下测其吸光值,并根据Trolox制成的标准曲线(Y=0.121X+5.684 4,R2=0.999 9)计算清除能力.
-
铁离子还原能力的测定参考Thaipong等[24]描述的方法,配制0.3 mol/L的醋酸缓冲液(pH值为3.6),10 mmoL/L TPTZ,20 mmoL/L FeCl3,按10∶1∶1的比例混合配制FRAP试剂. 取提取液0.4 mL与3.0 mL FRAP试剂混合,室温反应10 min,于593 nm波长下测定吸光值,并根据Trolox制成的标准曲线(Y=0.002 5X+0.105 2,R2=0.999)计算铁离子还原能力.
-
所有测定实验均独立重复3次. 采用Microsoft Excel 2021软件整理数据,采用SPSS 26.0软件、Origin 2021软件对数据进行分析和处理.
1.1. 供试材料
1.2. 方法
1.2.1. 取样及样品的制备
1.2.2. CGA质量分数与组分HPLC方法的建立
1.2.2.1. 色谱条件
1.2.2.2. 标准品溶液的制备
1.2.2.3. 系统性检验
1.2.3. 供试基因型的测定
1.2.4. 抗氧化能力的测定
1.2.4.1. ABTS自由基(SBTS+)清除能力测定
1.2.4.2. DPPH自由基清除能力测定
1.2.4.3. FRAP铁离子还原能力测定
1.2.5. 数据处理
-
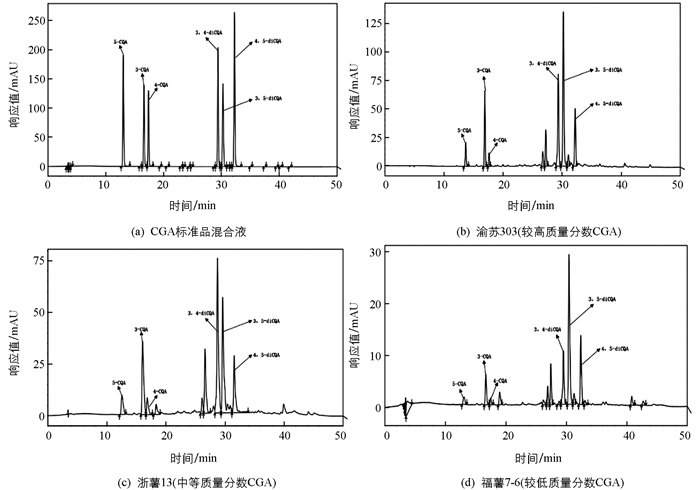
标准品溶液与供试品提取液色谱条件进样分析结果显示,在该色谱条件下,3-CQA,4-CQA,5-CQA,3,5-diCQA,3,4-diCQA和4,5-diCQA的组分间的分离和各个组分的基线分离均良好,供试品色谱峰与标准品色谱峰的保留时间一致,说明本方法专属性良好. 图 1为CGA标准品混合液和代表性基因型的HPLC图谱.
-
以浓度为横坐标,峰面积为纵坐标进行线性关系拟合,结果显示6个标准品在5~200 μg/mL浓度范围内线性关系良好(表 2).
-
取混合标准品溶液,按照1.2.2.1色谱条件连续进样6次,6种标准品峰面积的RSD值分别为0.08%,0.23%,0.46%,0.39%,0.14%,0.31%,表明仪器精密度良好.
-
取标准品溶液,分别于0,2,4,6,8,12,24 h进样10 μL,6种标准品峰面积的RSD值分别为0.09%,0.31%,0.48%,0.41%,0.12%,0.28%,表明标准品溶液在24 h内稳定性良好.
-
供试基因型叶片中5-CQA,3-CQA,4-CQA,3,4-diCQA,3,5-diCQA,4,5-diCQA组分和总CGA的质量分数统计分析结果表明,50个基因型叶片均有6种CGA组分,每100 g干物质中CGA总质量分数介于310.34~5 033.86 mg之间,变异系数为50.17%,平均值为2 398.48 mg,基因型间差异达到统计学意义(表 3). 以基因型S1(18-11-4),S2(18-11-5)和S3(161837)最高,每100 g干物质中CGA质量分数分别为5 033.86 mg,4 949.57 mg,4 941.80 mg,S49(福薯7-6)和S50(2019-1-15)最低,分别为504.65 mg,310.34 mg.
各CGA组分在总质量分数中占比的统计分析结果表明,3,5-diCQA,3,4-diCQA和3-CQA共3种CGA组分是50个基因型叶片绿原酸的主要成分,其质量分数在总质量分数中的占比平均值分别为35.36%,22.98%和21.54%,三者总占比为79.88%. 其次为4,5-diCQA,4-CQA和5-CQA占比较小,后两者不足10%(表 4).
-
CGA标准品不同单体的抗氧化能力测定结果表明,6种CGA单体的3种综合抗氧化能力由高到低依次为4,5-diCQA,3,5-diCQA,3,4-diCQA,3-CQA,4-CQA,5-CQA(表 5).
50个甘薯基因型叶片提取物抗氧化能力测定结果的统计分析表明,叶片提取物的ABTS,DPPH自由基清除能力和FRAP铁离子还原能力在50个基因型间差异有统计学意义,ABTS,DPPH自由基清除能力和FRAP铁离子还原能力最高基因型分别为最低基因型的5.06,7.08和9.75倍(表 6).
3种抗氧化指标排名前5的基因型及其Trolox当量(TE)见表 7,综合显示基因型S8,S10,S3和S1的综合抗氧化能力较强.
-
对50个基因型甘薯CGA各组分质量分数及在总质量分数中的占比,3种抗氧化能力共计15个指标进行的主成分分析(Principal components analysis,PCA)表明,6个CGA组分质量分数在总方差中贡献率达到95.48%,其中5-CQA特征值为7.86,方差贡献率达到52.39%. 15个指标可简化为特征值大于1的4个独立成分,方差贡献率分别为51.33%,18.68%,10.09%和8.15%,累计贡献率为88.25%. 主成分1主要是6种CGA组分质量分数指标,载荷均高于0.90,其次是ABTS,DPPH自由基清除能力和FARP铁离子还原能力指标,载荷值分别为0.84,0.81和0.95;主成分2主要是5-CQA,3-CQA,4-CAQ共3种CGA质量分数占比的正向载荷和3,5-diCQA的负向载荷,主成分3和主成分4分别是3,4-diCQA质量分数占比和4,5-diCQA质量分数占比的正向载荷. 50个基因型在这4个主成分上综合得分值介于0.10~3.13之间,排名前10的基因型由大到小依次为S1,S3,S4,S8,S5,S10,S7,S2,S9,S11,其分值介于1.27~3.13之间. 指标间相关性分析表明,抗氧化活性与CGA各组分质量分数的占比不相关,而与CGA各组分质量分数及总质量分数呈极显著正相关(数据略).
2.1. CGA质量分数与组分的HPLC方法建立
2.1.1. 适用性检验结果
2.1.2. 线性关系
2.1.3. 精密度实验
2.1.4. 稳定性实验
2.2. 甘薯叶片中的CGA各组分质量分数与占比分析
2.3. 抗氧化活性的测定
2.4. 主成分分析及相关性分析
-
酚类物质是植物界广泛存在的膳食抗氧化剂,甘薯块根和茎叶中的酚酸类化合物主要是CGA[25-26],包括3种单咖啡酰奎宁酸和3种二咖啡酰奎宁酸以及1种三咖啡酰奎宁酸(3,4,5-diCQA). Truong等[26]发现甘薯叶中的CGA质量分数最高,其次是薯皮、全根和薯肉组织. 本文研究结果表明,甘薯叶片中的3种单咖啡酰奎宁酸和3种二咖啡酰奎宁酸的质量分数及其总质量分数在基因型间差异有统计学意义,与Truong等[26]、Chen等[27]和Krochmal等[17]的研究结论一致. 本文中的基因型S1叶片CGA总质量分数每100 g干物质达到5 033.86 mg,是基因型S50的16.22倍,CGA等功能物质在品种之间的差异可能与在次级代谢物形成中起重要作用的遗传因素有关[24]. 50个基因型叶片中虽然均存在6种CGA组分,但是各个组分在总质量分数中的占比在基因型间差异有统计学意义. 总体而言,3,5-diCQA,3,4-diCQA和3-CQA 3种CGA组分是本文50个基因型叶片CGA的主要组分,这3种组分占比平均值分别为35.36%,22.98%和21.54%,三者总占比为79.88%,赵珊等[28]研究13个甘薯基因型的组分也发现,3,5-diCQA,3,4-diCQA和3-CQA为主要组分,三者总占比为79.05%.
CGA具有较强的抗氧化能力[25]. 已经有较多的研究表明甘薯叶片具有抗氧化活性和多种生理保健作用. 本文供试材料叶片提取物的ABTS,DPPH自由基清除能力和FARP铁离子还原能力在50个基因型之间差异有统计学意义,虽然这些基因型的抗氧化能力大小排序和CGA总质量分数以及各组分质量分数的排序不完全一致(乙醇提取物还含有其他非CGA活性成分),但是抗氧化能力与CGA质量分数之间总体上呈极显著正相关,这与傅玉凡等[29]、赵樱等[30]对于甘薯CGA的DPPH清除能力与其质量分数呈显著或极显著正相关的研究结果类似.
鉴于甘薯叶片遗弃浪费较大的产业现实以及可利用价值潜力,有必要开展富含CGA叶类甘薯新品种选育或淀粉、食用、加工类品种叶片品质改良的甘薯育种工作[31-32]. Ning等[33]研究发现CGA的广义遗传率高达0.84,因此进行富含CGA叶类甘薯新品种选育或品种改良理论上也是可行的. 种质资源创新与利用是甘薯遗传多样性拓展的重要途径,是品种改良的基础和保障. 许建华等[34-35]研究表明西蒙1号甘薯是药用甘薯品种,在临床上对多种出血性疾病及胰岛素非依赖型糖尿病有显著疗效,其茎叶乙醇提取物还具有一定的体内外抗肿瘤活性;Xi等[18]通过对渝紫薯7号和西蒙1号叶片中的多酚质量分数和抗氧化活性的对比研究表明,渝紫薯7号的多酚质量分数(甘薯茎叶中的多酚主要是CGA[25-26])与抗氧化能力分别是西蒙1号的1.18,1.28倍,是一个优异的富含CGA和高抗氧化能力的资源. 然而渝紫薯7号的CGA质量分数与抗氧化能力在本文50个基因型中分别排名第43位与第42位,因此本研究有机会筛选出较多的比渝紫薯7号更富含CGA和抗氧化能力更强的资源,如基因型18-11-4,161837,170407,18-6-33,18-11-5的CGA质量分数分别比渝紫薯7号高3.82,3.75,3.35,2.99,3.75倍,ABTS,DPPH自由基清除能力和FARP铁离子还原能力分别比渝紫薯7号最少高出1.29倍,1.84倍和1.82倍,因此,这些资源可用于高CGA、高抗氧化能力叶类利用与开发的甘薯品种的选育与改良工作. 例如,目前正在大力推广的高淀粉甘薯渝薯27叶片中CGA总质量分数每100 g干物质中含2 078.90 mg,可与叶富含CGA的淀粉资源18-11-5进行杂交育种筛选块根淀粉质量分数高、同时叶片也富含CGA的新品种,对渝薯27进行改良,实现薯块和叶片的综合利用,提高单位土地面积的甘薯产业效益.






 DownLoad:
DownLoad: