-
开放科学(资源服务)标识码(OSID):

-
慢性心力衰竭(chronic heart failure,CHF)是一种临床综合征疾病,是世界范围内心血管病死亡的主要原因之一[1-2]。尽管在过去的几十年里,药物和非药物治疗取得了重大进展,但CHF的高死亡率和再住院率仍居高不下[2-4]。CHF潜在的严重并发症和患病率,在全球都造成了沉重的经济负担[5-6]。因此,寻找CHF药物治疗新靶点迫在眉睫。
交感神经兴奋性增强是CHF等一系列疾病的主要病理特征。目前,CHF交感兴奋性亢进发病机制的研究进展主要包括外周和中枢两个方面。然而,外周采用肾交感切除术治疗CHF的长期有效性以及对心血管终点事件的影响还需大规模、多中心临床试验证实,仍处于临床前和探索阶段[7]。随着前期研究不断深入,CHF时交感神经激活中枢机制的特征引起学者们的广泛关注。下丘脑室旁核(paraventricular nucleus,PVN)作为重要的心血管交感神经紧张性活动整合中枢[8-9],在交感神经活动和多种神经体液调节过程中发挥关键作用。PVN内促炎性细胞因子[10]和微小核苷酸miR-133a[11]等内源性生物活性物质调节紊乱导致交感神经兴奋亢进,进而促进CHF发生发展。长链非编码RNA(long non-codingRNA,lncRNA)是一类长度大于200个核苷酸的转录本[12],参与染色质修饰[13]和调控蛋白质合成[14]等生物学过程。lncRNA不仅在胚胎发育[15]和神经元分化[16]等生理过程中发挥重要作用,而且与心肌肥厚[17]和帕金森病[18]等病理过程密切相关。然而,迄今为止中枢神经系统PVN内lncRNA是否参与CHF病理过程仍未见相关报道。有研究显示,PVN内调控2型小电导钙激活钾通道蛋白(small-conductance Ca2+-activated K+ channel 2,SK2)表达下调与CHF交感兴奋亢进密切相关[19],但SK通道下调的确切机制仍不明确。
本研究预实验结果显示,CHF大鼠PVN组织和血管紧张素Ⅱ(angiotension Ⅱ,ANGII)孵育的原代培养下丘脑神经元内长链非编码RNA LOC116886540表达显著下调,并将其命名为心力衰竭相关调节剂(heart failure associated regulator,HFAR),lncRNA HFAR由1 973个碱基构成,其编码基因位于17号常染色体NC 046170.1(28977446~28979418)上,基因ID 116886540,转录本为XR_004386110.1。据此,提出假设:CHF状态下PVN中过表达lncRNA HFAR可以使SK2表达上调,抑制交感神经兴奋亢进,最终改善CHF症状。
HTML
-
荧光二抗(购自美国LI-COR公司);一抗(分别购于Sigma-Aldrich©、Aomone lab和Abcam公司);小动物超声系统(Fugifilm);PowerLab数据采集分析系(ADInstruments)等。
-
6~8周SD雄性大鼠,体质量为200~350 g,由北京维通利华实验动物技术有限公司购入,合格证号:SCXK(京)-2021-0011。
-
实验采用结扎冠状动脉左前降支法构建心衰大鼠模型,将大鼠随机分为Sham组和CHF组。①构建CHF组大鼠模型,腹腔注射复合麻醉剂,仰卧位固定大鼠,手术开胸暴露心脏,结扎冠状动脉左前降支,观察结扎下方左心室壁颜色变白,心电图有明显的缺血表现时,断开呼吸机,待大鼠恢复自主呼吸后,轻轻拔掉气管插管,并立即缝合气管和颈部。② Sham组冠状动脉左前降支仅穿线不结扎,其他操作与CHF组相同。术后大鼠每天皮下注射0.2 mg/kg美洛昔康和肌肉注射20万U青霉素,持续3 d,进行抗感染治疗。4周后鉴定心衰模型是否成功。
-
大鼠术后4周,待腹主动脉取血完成后,迅速摘取大鼠心脏,自结扎线以下将其切成5块厚度约1 mm的均匀切片,迅速置于1%的TTC溶液中染色约15 min后观察拍照。红色区域为正常的心肌组织,梗死区域呈现白色,以梗死区域面积/心脏面积的比值代表梗死范围。
-
大鼠术后4周,利用装有异氟烷的气麻机持续麻醉大鼠后,仰卧位固定在大鼠鼠板上,将胸部左侧胸前区进行广泛备皮处理。采用Vevo 3100超声仪对大鼠进行胸部超声心动图检查,配有MX250探头,M型记录,超声心动图系统评估左心室功能。超声心动图参数包括左心室舒张末期内径(left ventricular internal diameter diastolic,LVIDd)等。
-
腹腔注射混合麻醉剂,通过股动脉插管测量平均动脉压(mean arterial pressure,MAP)和心率(heart rate,HR)。气管插管后,静脉慢性输入加拉典铵25 mg/(kg·h)麻痹大鼠呼吸肌,并用室内高氧空气进行人工通气。分离颈部组织,暴露出右侧颈总动脉,经颈动脉插管至左心室,术后稳定15 min后,测定其左心室舒张末期压力(left ventricular end-diastolic pressure,LVEDP),左心室最大收缩速率(dp/dt Max)和左心室最大舒张速率(dp/dt Min)。待血流动力学指标检测完毕,用过量氯化钾安乐死大鼠,然后剪开大鼠胸腔,取出心脏和肺脏称质量。
-
麻醉大鼠,将其固定在脑立体定位仪上,调整头骨水平。通过颅骨钻打孔暴露硬脑膜,根据立体定位坐标将金属套管尖端下降至PVN中,利用微量注射器注射病毒。对4周造模成功后的大鼠进行人工脑脊液组(NULL)、HFAR(重组腺相关病毒血清型2/9-HFAR过表达组)和/或NC HFAR(重组腺相关病毒血清型2/9-HFAR过表达阴性对照组)病毒微量注射,每次注射完毕留针10 min。注射结束后缝合皮肤,术后3 d连续每天皮下注射0.2 mg/kg美洛昔康和肌肉注射20万U青霉素,避免感染发炎。第5周进行HFAR ASO(重组腺相关病毒血清型2/9-HFAR敲减组)和/或NC HFAR ASO(重组腺相关病毒血清型2/9-HFAR敲减阴性对照组)病毒微量注射,上述全部病毒载体微量注射均采用PVN双侧注射。单侧注射体积为50 nL,病毒滴度为1×1015v.g./L,于9周后记录大鼠心功能、交感驱动、血流动力学指标并在心脏采血。上述实验结束时,注射戊巴比妥钠安乐死,将大鼠灌流取脑,免疫荧光检测PVN内SK2蛋白表达量,通过激光聚焦显微镜扫描鉴定显微注射位点。
-
靠近脊椎左侧开口,打开腹腔,从周围组织中分离出左肾交感神经并固定在银丝电极上,滴加37 ℃石蜡油于神经上起绝缘和湿润保护作用。经四通道交流/直流差分放大器放大1 000倍后,记录肾交感神经活动(renal sympathetic nerve activity,RSNA),用PowerLab数据分析处理系统进行积分处理,同步实时记录原始RSNA数值和积分RSNA数值。为记录噪音水平值,在实验结束时注射氯化钾,排除肾交感神经传出活动后记录的值即为噪音水平。RSNA积分值与噪音积分值的差值即为实际肾交感神经传出活动的积分值。
-
大鼠在麻醉状态下,通过心脏穿刺收集大鼠血液样本。血浆去甲肾上腺素(Norepinephrine,NE)水平用ELISA试剂盒按照厂家说明书进行检测,结果由酶标仪读出。
-
将大鼠用异氟烷麻醉后,固定在脑立体定位仪上,用颅骨钻打孔暴露小脑。将玻璃微电极尖端下降至延髓头端腹外侧(rostral ventrolateral medulla,RVLM)区域,双侧RVLM各显微注射50 nL霍乱毒素B亚基(CTB)。每次进样结束后,留针20 min。连续3 d皮下注射美洛昔康和肌肉注射青霉素,以镇痛和防止感染。
-
注射重组腺相关病毒术后4周,随机选取6只大鼠进行研究。先将鼠脑切片用PBS洗涤3次,每次10 min,再把切片放在37 ℃培养箱中封闭3 h后,重复上述洗涤方法,随后加入一抗,放在冰箱4 ℃孵育48 h。最后,用PBS冲洗并在避光条件下加入荧光标记的二抗,室温孵育1 h,再用PBS洗净后进行贴片处理,随后采用激光共焦显微镜进行扫描,统计SK2表达量。
-
冰浴状态下,切取2只大鼠PVN组织混为一个样本。将冷冻的脑组织按照1∶10体积放置在含蛋白酶抑制剂的裂解缓冲液中,匀浆3 min,再将其放在高速冷冻离心机中以4 ℃、12 000 r/min离心5 min。收集上清液,用Bio-Rad DC蛋白测定试剂盒测定蛋白浓度。等量蛋白质样品利用Buffer煮沸后,采用SDS-PAGE(7.5%)分离,将蛋白质从凝胶转移到PVDF膜上,并用5%脱脂奶粉室温封闭1 h,洗涤3次后用兔Anti-SK2 Antibody和小鼠抗GAPDH在4 ℃孵育24 h,24 h后再洗涤3次。用山羊抗兔680RD和山羊抗小鼠800CW荧光二抗于暗室中室温下孵育1 h,洗涤3次后用双色红外激光成像系统Odyssey CLx扫膜检测。
-
无菌操作,将恒温37 ℃、5%CO2-95%空气培养箱中分离出的新生大鼠下丘脑神经细胞,用0.25%胰蛋白酶消化。离心后,将消化后的细胞悬浮,然后在含有10%胎牛血清,100 U/mL青霉素和100 μ g/mL链霉素的DMEM培养基中培养,取对数生长期的细胞用于后续qRT-PCR检测。
-
用Trizol法提取下丘脑组织总RNA,通过紫外分光光度计测定浓度,在260 nm处和280 nm处测定吸光度值,并计算浓度和纯度。取总RNA 0.5 μg置于逆转录仪中进行逆转录,再以cDNA为模板,按合成试剂盒说明书进行扩增。以GAPDH作为内参,利用2-ΔΔCt方法计算表达量相对水平。
-
数据使用10.1.2版GraphPad Prism软件进行评估,并以平均值±标准误(SEM)表示。采用Shapiro-Wilk方法评估数据的正态性,数据符合正态分布。采用双尾非配对t-检验评估两组之间的显著性差异,并利用单因素方差分析(One-way ANOVA)和Bonferroni检验进行多组间比较,*表示p<0.05水平差异具有统计学意义,**表示p<0.01水平差异具有统计学意义,***表示p<0.001水平差异具有统计学意义。
1.1. 材料与试剂
1.2. 方法
1.2.1. 实验动物
1.2.2. CHF大鼠模型
1.2.3. 心肌梗死面积检测
1.2.4. 超声心动图评估心脏功能
1.2.5. 血流动力学指标和解剖学检测
1.2.6. PVN微量注射
1.2.7. 肾交感神经活动记录
1.2.8. 血浆NE水平检测
1.2.9. PVN-RVLM神经元的逆行标记
1.2.10. 免疫荧光检测
1.2.11. 蛋白印迹分析
1.2.12. 新生乳鼠下丘脑原代培养
1.2.13. 实时荧光定量PCR
1.2.14. 统计与分析
-
与Sham组相比,CHF组心肌梗死面积均显著升高(p<0.01),左心室射血分数(ejection fraction,EF)和短轴缩短率(fractional shortening,FS)均显著下降(p<0.01),且EF≤45%(表 1)。结果提示:慢性心肌缺血诱导大鼠心力衰竭模型制备成功。
-
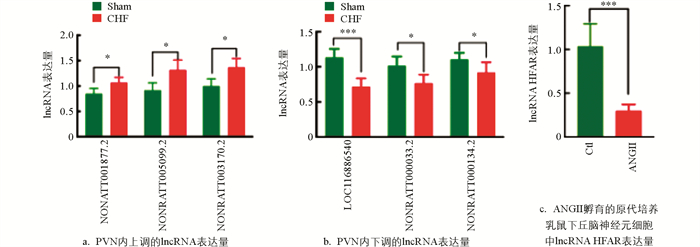
应用qRT-PCR方法进行验证,与Sham组相比,CHF组下丘脑PVN内3个表达上调显著的lncRNA分别为NONRATT001877.2、NONRATT005099.2和NONRATT003170.2(p<0.05)(图 1a);3个表达下调显著的lncRNA分别为LOC116886540(p<0.001)、NONRATT000033.2(p<0.05)和NONRATT000134.2(p<0.05)(图 1b)。与正常神经细胞相比,lncRNA HFAR在ANGII孵育的原代培养乳鼠下丘脑神经细胞中表达显著降低(p<0.001)(图 1c)。结果提示:PVN内下调的lncRNA HFAR可能与CHF大鼠交感神经兴奋亢进有关。
-
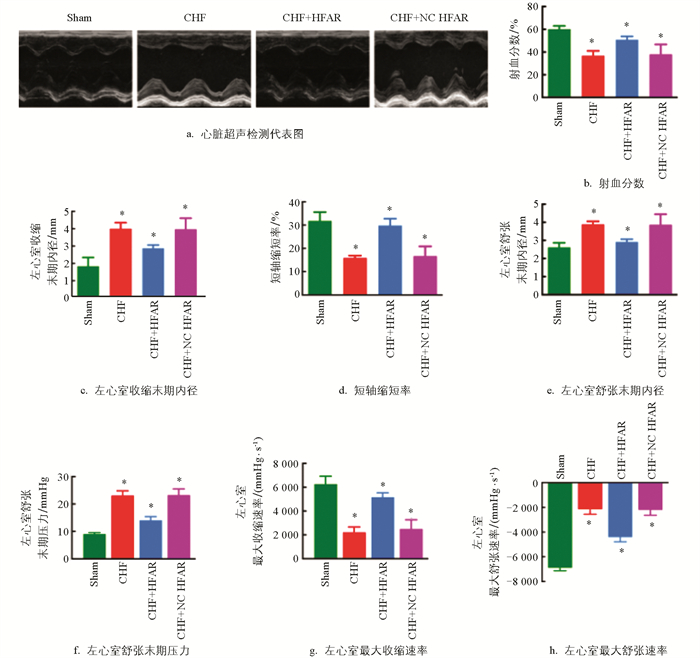
与假手术组相比,CHF组大鼠心脏EF、FS、左心室最大收缩速率和左心室最大舒张速率显著下降,左心室收缩末期内径(left ventricular internal diameter systolic,LVIDs)和LVIDd明显增加(p<0.05)。PVN内过表达lncRNA HFAR可明显逆转CHF大鼠EF和FS下降,LVEDP、LVIDs和LVIDd的增加(p<0.05)(图 2a-h)。结果提示:PVN内过表达lncRNA HFAR可明显改善CHF大鼠心功能。
-
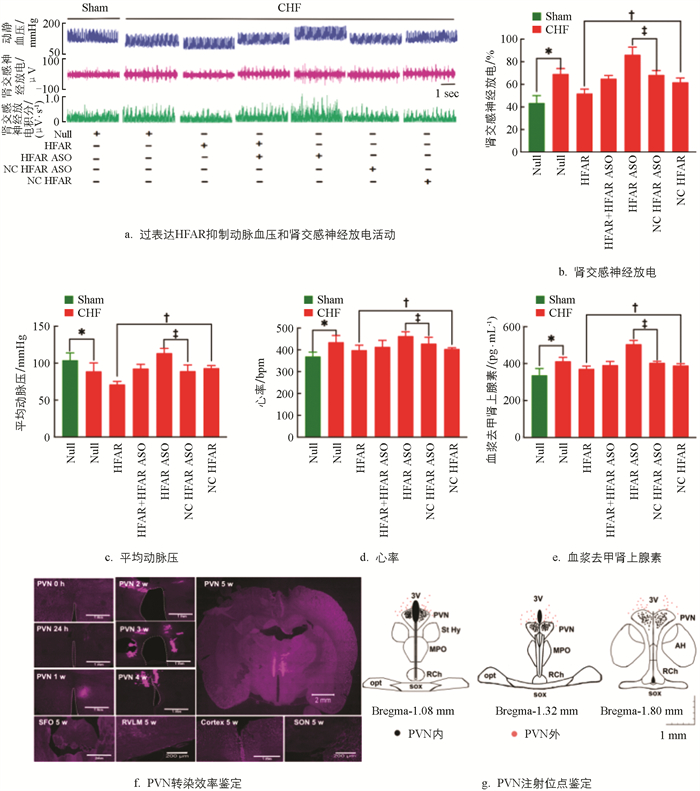
与Sham组相比,CHF组大鼠的交感神经驱动指标RSNA、HR和NE水平显著升高,MAP显著降低(p<0.05)。与CHF+NC HFAR组相比,CHF+HFAR组大鼠RSNA、HR和NE水平显著升高,MAP显著降低(p<0.05);与CHF+NC HFAR ASO组相比,CHF+HFAR ASO组大鼠RSNA、HR、MAP和NE水平显著升高。结果提示:PVN内下调的lncRNA HFAR可增强CHF大鼠交感神经激活作用(图 3a-e)。
在大鼠PVN内注射rAAV2/9-hSyn-mCherry-HFAR病毒后,24 h内未见表达,24 h至5周时间内,转染效率呈时间依赖性增加,5周时转染效率达顶峰。然而,在穹窿下器官、RVLM、大脑皮层和视上核区域均未见红色荧光蛋白表达。结果提示:5周内HFAR病毒转染具有PVN核团特异性(图 3f)。参考大鼠脑立体定位图谱,鉴定注射位点,通过观察红色荧光蛋白表达区域是否落在PVN内,判定病毒注射是否成功。在交感神经驱动指标记录实验中,双侧核团注射60次,其中42次注射在PVN内(图 3g)。
-
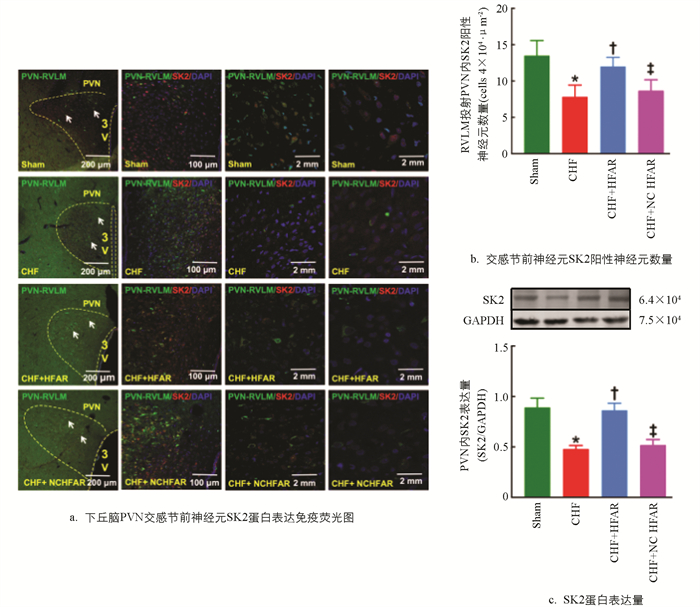
与假手术组相比,CHF组大鼠PVN内交感节前神经元SK2蛋白表达量显著下调;与CHF+NC HFAR组相比,PVN内过表达lncRNA HFAR可明显上调CHF组SK2蛋白表达量(图 4a-c)。结果提示:PVN内下调的lncRNA HFAR可导致交感节前神经元SK2蛋白表达量降低,与CHF状态下交感神经兴奋性亢进有关。
2.1. 解剖学、血流动力学、心功能和血液生化指标检测
2.2. Sham组与CHF组PVN内差异lncRNA的筛选及鉴定
2.3. PVN内过表达lncRNA HFAR对CHF大鼠心功能指标的影响
2.4. PVN内过表达lncRNA HFAR对CHF组大鼠交感神经驱动指标的影响
2.5. PVN内过表达lncRNA HFAR对CHF大鼠PVN内SK2蛋白表达的影响
-
本研究应用qRT-PCR方法发现CHF大鼠PVN组织中lncRNA HFAR极显著下调,过表达lncRNA HFAR显著改善CHF大鼠心脏功能和抑制交感神经兴奋性增加。周壮[20]证实在腰椎间盘突出症大鼠PVN内lncRNA NORATTO00069.2、NONRATT005099.2、NONRATT000033.2和NONRATT000134.2表达失衡,可能与神经病理性疼痛有关。Zhang等[21]研究发现在应激性高血压大鼠RVLM中lncRNA INPP5F显著下调,过表达lncRNA INPP5F显著改善了应激诱导的血压升高和交感神经兴奋性增加。以上结果提示:在中枢内lncRNAs表达失衡可以调节交感传出神经元活动,从而参与CHF、高血压和疼痛的发生发展。
SK通道至少包括SK1、SK2和SK3共3种亚型,其中SK2对Apamin亲和力最高,且神经系统中主要表达SK2和SK3两种亚型。本研究发现CHF大鼠PVN组织中SK2通道蛋白表达显著下调,可能与交感神经兴奋性增加有关,这与李晓燕等[19]的研究结果一致。另有研究显示,CHF大鼠视上核中SK2和SK3通道mRNA表达和功能显著降低,可能是导致大细胞神经元兴奋性增加的重要机制[22]。Chen等[23]发现ANGII和高盐饮食诱导的高血压大鼠PVN-RVLM神经元中SK电流减少,导致中后超极化电位减弱和神经元兴奋性增加。Chapp等[24]研究表明,长期高盐摄入的大鼠PVN-RVLM交感节前神经元SK电流降低与PVN神经元兴奋性增加有关。Pachuau等[25]发现自发性高血压大鼠PVN-IML(intermediolateral cell column,IML)交感节前神经元中SK通道功能减弱导致PVN神经元兴奋性增加,与PVN内SK3通道mRNA和蛋白质表达量无关。Larson等[26]报道PVN内SK通道功能降低引起ANGⅡ-盐性高血压相关的交感神经兴奋,与PVN内SK1、SK2和SK3通道蛋白表达量无关。综上结果提示:在下丘脑PVN内SK通道的表达失衡或功能紊乱可以调节交感传出神经兴奋和PVN内神经元活动,从而参与CHF和高血压的发生发展。
本研究发现lncRNA HFAR可直接上调SK2蛋白表达抑制CHF大鼠交感神经兴奋亢进,其机制可能与lncRNA直接影响SK通道的膜运输过程有关。Ma等[27]报道lncRNA可通过突触素2b蛋白下调α-氨基-3-羟基-5-甲基-4-异唑丙酸受体(AMPAR)表达,从而增强突触可塑性和影响社会等级。结果提示:lncRNA对膜通道蛋白表达量的影响与其参与调节配体门控通道的膜运输过程有关。另有研究发现,lncRNA DACH1不仅在心力衰竭小鼠中通过泛素化修饰下调内质网钙ATP酶2蛋白表达损害心脏功能[28],而且在糖尿病心肌病(DCM)直接与沉默信息调节因子相关酶3 (SIRT3)结合并促进其泛素化降解,进而促进小鼠心脏线粒体氧化损伤和细胞凋亡[29]。Zhang等[30]报道,在心肌梗死小鼠中lncRNA ZFAS1可通过磷酸化作用直接下调内质网钙ATP酶2蛋白表达,从而使心脏收缩功能受损。综上所述,lncRNA可通过影响离子通道的膜运输、泛素化和磷酸化修饰等过程调节蛋白质表达量,参与神经系统和心血管系统生理和病理过程。
-
本研究首次发现CHF大鼠下丘脑PVN内lncRNA HFAR发生显著下调,并探索了lncRNA HFAR对CHF大鼠心脏功能、HR、MAP、RSNA、血浆NE和PVN内SK2通道表达的影响。研究显示,过表达lncRNA HFAR显著改善了CHF大鼠心脏功能、抑制交感驱动指标并上调CHF大鼠PVN内SK2蛋白表达。结果提示:PVN中下调的lncRNA HFAR通过抑制SK2通道蛋白表达导致CHF状态下交感兴奋性亢进。






 DownLoad:
DownLoad: