-
开放科学(资源服务)标识码(OSID):

-
抗生素能促进畜禽生长和预防疾病,但长期使用可能会导致耐药性的产生,其滥用还会引起肠道菌群失衡、免疫和生产性能下降、环境污染等,因此,绿色、安全和可持续的抗生素替代品应运而生,对减少抗生素使用和环境污染至关重要[1].
益生菌是一种有益于宿主的活性微生物,通过改善肠道微生物组成、加强肠道上皮屏障功能、调节免疫系统、对黏膜和上皮的竞争性黏附等机制影响宿主胃肠道功能,从而改善畜禽健康和提高生产力[2-4]. 研究指出,粪肠球菌可改善哺乳母猪表观消化率,增加其仔猪出生质量、平均日增质量和料质量比,并且还能提高肉鸡饲料转化率[5-7]. 乳酸杆菌可增加断奶仔猪肠道微生物多样性和丰富度,减弱回肠炎症基因表达,促进肠道发育[8]. 枯草芽孢杆菌能提高仔猪回肠甘油三酯、脂肪酶、淀粉酶含量和绒隐比,还能在瘤胃中建立有益菌群,提高小牛断奶后的饲料利用率,并减少甲烷的产生[9-10]. 双歧杆菌可增加肉鸡血清超氧化物歧化酶和免疫球蛋白含量,改善抗氧化状态和免疫应答[11]. 丁酸梭菌有利于改善肉鸡的胫骨发育和对钙、磷的吸收利用[12]. 屎肠球菌可改善肉鸡肠道菌群结构,提高短链脂肪酸产生菌的相对丰度,促进肠道的磷吸收和骨形成代谢活动[13]. 嗜酸乳杆菌液则可显著降低蛋鸡残破蛋比例以及血清和蛋黄胆固醇含量[14].
以上研究表明单一益生菌对畜禽动物有积极作用,而多种益生菌组合能更有效地发挥相互之间的协同作用,在肠道健康和生产性能之间创造更有利的平衡[3, 15],可更大限度地发挥益生菌的优势,且已在畜禽生产中得到了广泛应用[16]. Liu等[17]研究显示与单一粪肠球菌相比,干酪乳杆菌和粪肠球菌的复合菌能显著提高仔猪IgG含量,并降低死亡率和7日龄腹泻率. Yu等[18]研究指出与单一丁梭状芽孢杆菌或枯草芽孢杆菌相比,二者的复合菌显著提高了扬州鹅空肠黏膜T-SOD含量和回肠绒毛高度. Lema等[19]研究也表明L. acidophulus和S. faecium的复合菌与其单一菌比较,复合菌能显著改善羔羊平均日增质量和料质量比,且显著降低粪便中出血性大肠杆菌数量.
麻羽肉鸡是中国特有的肉鸡品种,具有肉质细嫩、营养价值高、适应性强、生长速度快、抗病性强等特点,深受广大消费者的喜爱. 本试验探讨了饲粮中添加不同水平复合菌制剂对麻羽肉鸡血清代谢、生长、消化、免疫性能和肠道微生物的影响,旨在为复合菌制剂在麻羽肉鸡上的应用提供理论依据.
HTML
-
复合菌制剂主要由粪肠球菌(≥50.0×108 CFU/g),丁酸梭菌(≥2.0×108 CFU/g),屎肠球菌(≥0.1×108 CFU/g)和嗜酸乳杆菌(≥1.0×108 CFU/g)组成.
选取240只体况健康的1日龄麻羽肉鸡(公、母鸡比例为1∶1),随机分为4组,每组6个重复,每个重复10只,分别在基础饲粮中添加0(对照),0.05%,0.1%和0.2%的复合菌制剂. 所有试验肉鸡在同一环境下采用3层立笼式饲养,自由采食和饮水,饲养作息、免疫、卫生消毒等程序按照标准养殖制度执行,试验期共90 d. 参照美国国家科学院(1994)制定的鸡的营养需要标准配制基础饲粮,其组成及营养水平如表 1.
-
试验结束前4 d,每个重复随机选取3只麻羽肉鸡饲养于代谢笼,采用全收粪法连续收集3 d粪便. 试验结束后,每个重复选取4只接近本栏平均质量的鸡,称质量后使用真空采血管(不含抗凝剂)采集颈静脉血10 mL,静置20 min后3 500 r/min离心10 min后取上清液,-20 ℃下保存备用;屠宰后迅速分离出肝脏、胰腺、肌胃、腺胃、脾脏、胸腺、法氏囊、十二指肠、空肠和回肠,并轻轻挤出十二指肠、空肠和回肠内容物,生理盐水冲洗干净,滤纸吸干水分;取盲肠内容物并汇合成1个样品-80 ℃冻存于无菌管中.
-
参照NY/T 823-2020《家禽生产性能名词术语和度量计算方法》[20]测定生长性能,即每天早上喂料前记录耗料量,试验开始和结束当天早上对每只鸡空腹称质量,计算平均日采食量、平均日增质量和料质量比.
-
利用全自动血液生化分析仪(OTA-400)测定血清总蛋白、总胆固醇、甘油三酯、高(低)密度脂蛋白、谷丙转氨酶、谷草转氨酶、碱性磷酸酶和肌酸激酶的含量;以溶酶小球菌体细胞为底物,采用比浊法测定溶菌酶活性;使用单克隆抗体免疫比浊法测定免疫球蛋白A(IgA)、免疫球蛋白G(IgG)和免疫球蛋白M(IgM)活性.
-
称取肝脏、胰腺、肌胃(去除肌内金)、腺胃、脾脏、胸腺、法氏囊、十二指肠、空肠和回肠质量后,计算器官指数(I):
式中:m1为器官鲜质量;m2为活体质量.
-
记录代谢笼每只鸡的采食量,清除所收集粪便中的羽毛等杂物后称质量,并按照每100 g粪样5 mL加入10%硫酸固氮. 将饲料原料、剩料和粪便于65 ℃烘干48 h,回潮24 h称质量,粉碎机过40目筛并充分混匀后待测. 总能量(GE)采用燃烧法(德国IKA2000全自动氧弹测定仪)测定;粗蛋白采用凯氏定氮法(GB/T 6432-2018)测定;钙(Ca)采用EDTA络合滴定法(GB/T 6436-2018)测定;磷(P)采用分光光度法(GB/T 6437-2018)测定.
-
采集十二指肠、空肠和回肠各肠段约5 cm,Tris缓冲盐水冲洗肠段,4%多聚甲醛固定,石蜡包埋,切成5 μm薄片,苏木精&伊红(H&E)染色,然后在22倍放大镜下拍照;采用Image Pro Plus 6.0测量绒毛高度、隐窝深度和黏膜厚度. 每个样品测3个重复值,并计算绒隐比.
-
利用QIAamp DNA Stool Mini Kit(QIAGEN,德国)提取盲肠微生物总DNA,扩增16S rRNA V3-V4区(338F:5′-ACTCCTACGGGAGGCAGCAG-3′,5′端带条形码标记;806R:5′-GGACTACHVGGGTWTCTAAT-3′). PCR扩增产物用QuantiFluorTM-ST蓝色荧光定量系统检测,根据测序量要求按比例混合构建文库进行Miseq测序. 对双端序列数据进行拼接、筛选、质控、过滤等一系列处理后,在97%相似度下利用Usearch 7.1对序列聚类,得到分类操作单元(OTUs),然后用RDP Classifer将OTUs代表序列与数据库Silva进行比对(置信度阈值为0.6).
-
α多样性以物种丰富度指数(Chaol和ACE)和多样性指数(Shannon和Simpson)表示;β多样性通过偏最小二乘回归分析法(PLS-DA)进行可视化,并通过计算非加权UniFrac距离矩阵制作组间差异盒状图. 使用SPSS 22.0软件进行方差分析,如存在统计学意义,用Duncan法进行多重比较. 使用线性和二项式回归分析评估复合菌制剂添加水平的影响,结果以x±s表示,p<0.05为差异有统计学意义.
1.1. 试验材料
1.2. 样品采集
1.3. 指标测定
1.3.1. 生长性能
1.3.2. 血清指标
1.3.3. 器官指数
1.3.4. 消化代谢
1.3.5. 肠道形态
1.3.6. 肠道微生物
1.4. 数据分析
-
由表 2可知,与对照相比,添加复合菌显著提高了麻羽肉鸡末质量(p<0.05),且0.05%和0.1%复合菌制剂组显著提高了麻羽肉鸡平均日增质量(p<0.05),而0.1%复合菌制剂组又显著高于0.05%复合菌制剂组(p<0.05);同时,0.1%复合菌制剂组显著提高了麻羽肉鸡的采食量(p<0.05).
-
由表 3可知,与对照相比,0.05%和0.2%复合菌制剂组显著降低了麻羽肉鸡谷草转氨酶活性(p<0.05),而总蛋白和高密度脂蛋白质量浓度在0.1%复合菌制剂组显著升高(p<0.05),碱性磷酸酶活性在0.1%复合菌制剂组显著降低(p<0.05). 此外,随复合菌制剂量的增加,甘油三酯质量浓度呈显著的二项式升高趋势(p=0.04),且0.1%复合菌制剂组质量浓度达到最高.
-
由表 4可知,与对照相比,0.1%复合菌制剂组显著提高了麻羽肉鸡胰腺、空肠和回肠指数(p<0.05),而0.05%和0.2%复合菌制剂组差异无统计学意义(p>0.05);复合菌制剂组均显著提高了麻羽肉鸡钙和磷消化率(p<0.05),其中0.1%和0.2%复合菌制剂组之间差异无统计学意义(p>0.05). 此外,随复合菌制剂量的增加,粗蛋白消化率呈显著升高的趋势(线性:p=0.05;二项式:p=0.02),且0.2%复合菌制剂组消化率达到最高.
-
由表 5可知,与对照相比,0.05%复合菌制剂组显著提高了空肠和回肠绒毛高度、隐窝深度和黏膜厚度(p<0.05),但显著降低了绒隐比(p<0.05);0.1%复合菌制剂组显著提高了绒毛高度和黏膜厚度(p<0.05);0.2%复合菌制剂组显著降低了绒毛高度和黏膜厚度(p<0.05). 此外,随复合菌制剂量的增加,回肠绒毛高度呈显著的二项式升高趋势(p<0.05),且0.1%复合菌制剂组达到最高.
-
由表 6可知,与对照相比,0.1%复合菌制剂组显著提高了麻羽肉鸡胸腺和法氏囊指数(p<0.05),而0.05%和0.2%复合菌制剂组间差异无统计学意义(p>0.05);同时,0.1%复合菌制剂组显著提高了IgG和溶菌酶质量浓度(p<0.05),而0.2%复合菌制剂组差异无统计学意义(p>0.05). 此外,随复合菌制剂量的增加,IgG质量浓度呈显著的二项式升高趋势(p=0.05),且0.1%复合菌制剂组最高.
-
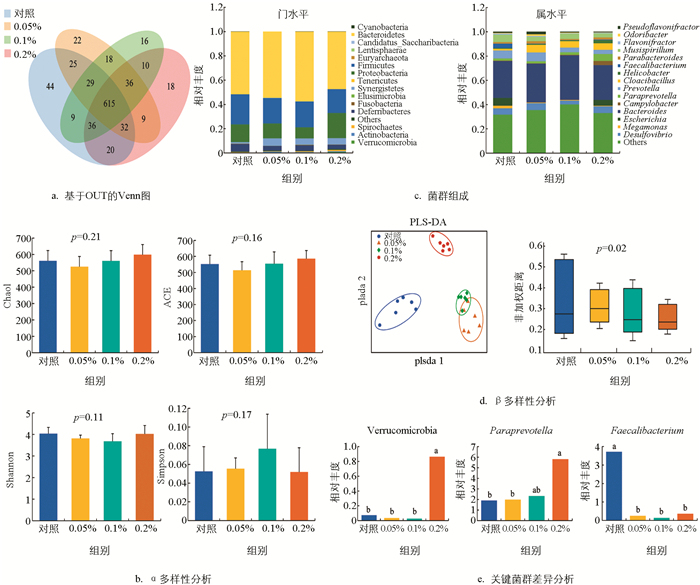
不同水平复合菌制剂饲喂麻羽肉鸡的肠道微生物测序序列文库覆盖率均大于97.48%,比对后共得到939个OTUs(对照为810个,0.05%为786个,0.1%为769个,0.2%为776个),其中共享615个(图 1a). α多样性分析表明,添加不同水平复合菌制剂对麻羽肉鸡肠道微生物丰富度和多样性影响无统计学意义(p>0.05,图 1b). 分类学结果显示:对照有16门112属,0.05%复合菌制剂组有14门93属,0.1%复合菌制剂组有15门93属,0.2%复合菌制剂组有15门93属. 门水平上,主要由拟杆菌门(Bacteroidetes)(对照:51.62%;0.05%:54.50%;0.1%:57.51%;0.2%:47.41%),厚壁菌门(Firmicutes)(对照:24.80%;0.05%:21.00%;0.1%:21.27%;0.2%:19.58%),变形杆菌门(Proteobacteria)(对照:14.55%;0.05%:12.25%;0.1%:9.01%;0.2%:20.71%)等组成;属水平上,相对丰度占比1%以上的共有菌有Bacteroides,Mucispirillum,Desulfovibrio,Cloacibacillus,Prevotella和Paraprevotella(图 1c). β多样性分析显示肠道微生物菌群结构根据复合菌制剂水平发生明显聚类,且组间差异有统计学意义(p=0.02,图 1d). 此外,关键菌群的差异分析显示0.2%复合菌制剂组显著提高了疣微菌门(Verrucomicrobia)和帕拉普氏菌属(Paraprevotella)的相对丰度(p<0.05),而复合菌制剂组则显著降低了普拉梭菌属(Faecalibacterium)的相对丰度(图 1e).
2.1. 生长性能
2.2. 血清代谢
2.3. 消化性能
2.4. 肠道形态
2.5. 免疫球蛋白及器官指数
2.6. 肠道微生物
-
本研究结果表明,0.05%和0.1%复合菌制剂组显著提高了麻羽肉鸡平均日增质量,适量的复合菌能促进麻羽肉鸡的生长. 复合菌制剂主要由粪肠球菌、屎肠球菌、嗜酸乳杆菌和丁酸梭菌构成,前三者均属于乳酸菌,能诱导盲肠中乙酸、丁酸等短链脂肪酸(SCFA)的产生,而丁酸梭菌的代谢产物即为丁酸. SCFA既是宿主的能量来源,还能增强上皮细胞的屏障功能,调节黏蛋白并促进脂肪基因表达,加强脂肪沉积,进而提高生长性能[21-23]. 盲肠的SCFA含量升高则会降低其pH值,改善肠道蠕动和消化酶分泌,促进饲料消化和营养物质吸收. 此外,前人研究也证实无论是单一的粪肠球菌、丁酸梭菌、屎肠球菌、嗜酸乳杆菌还是其复合菌均能不同程度地改善家禽生长性能,且复合菌效果更好[24-25],表明复合菌之间存在一定的协同黏附作用,对构建良好的肠道菌群体系有积极作用,可为有益菌群的定植提供有利条件来促进肠道健康[3]. 0.2%复合菌制剂组则没有体现出对生长性能有更好的效果,主要原因可能是0.2%复合菌制剂组降低了肠道绒毛高度、绒隐比和黏膜厚度,影响了麻羽肉鸡对营养物质的消化与吸收.
谷草转氨酶反映肝脏的组织渗透性,是评价肝损伤的重要指标[26-27]. 本研究中,复合菌制剂的添加降低了麻羽肉鸡血清中谷草转氨酶的活性,表明复合菌制剂改善了麻羽肉鸡肝功能,主要得益于丁酸梭菌可激活肝脏受体信号通路,促进淋巴细胞发育和分化,降低促炎症相关基因的表达,并增加抗炎症和抗氧化相关基因的表达[28]. Xia等[29]研究显示嗜黏杆菌可促进SCFA合成,减轻肝脏的氧化应激和炎症来降低对肝的损伤. Chen等[26]研究则指出复合菌(Lactobacillus agilis,Lactobacillus reuteri)对AA肉鸡血清谷丙转氨酶无影响,说明不同复合菌组成及添加水平具有不同影响,应该谨慎比较不同的研究结果. 此外,0.1%复合菌制剂组显著降低了血清碱性磷酸酶的活性,显著提高了高密度脂蛋白含量,其含量高低反映了机体的健康状态,比如碱性磷酸酶是肝和骨骼相关疾病的重要血清指标[30],而高密度脂蛋白具有保护心血管的作用[31],因此适量的复合菌对麻羽肉鸡健康有积极影响. 其可能原因是复合菌所含乳酸菌的抗氧化作用可缓解肠细胞的氧化应激和炎症,使高密度脂蛋白颗粒具有更高的生物质量,有助于改善其在机体内的新陈代谢. 适量的复合菌在促进蛋白质吸收、代谢和沉积方面也具有积极作用. 本研究中0.1%复合菌制剂组显著提高了血清总蛋白的含量. 复合菌能刺激机体产生更多的蛋白酶,提高饲料蛋白质分解成氨基酸的速率,增强氨基酸间的相互竞争,进而促进机体对氨基酸的利用并加速蛋白质的合成. 复合菌制剂的添加对甘油三酯含量影响无统计学意义,但其含量则与复合菌制剂水平呈显著的二项式关系,且0.1%复合菌制剂组达到最高,进一步表明麻羽肉鸡在0.1%复合菌制剂饲喂后脂肪沉积能力最强.
-
消化器官的生长发育指数在一定程度上能反映机体的消化性能和健康状况[32]. 本研究中,0.1%复合菌制剂组提高了麻羽肉鸡胰腺、空肠和回肠指数,且改善了肠道绒毛高度、绒隐比和黏膜厚度. 这与Salim等[33]研究结果相似,复合菌(L. reuteri,Bacillus subtilis,Saccharomyces cerevisiae)显著提高了白羽肉鸡回肠绒毛高度和黏膜厚度. 此外,二项式回归分析也表明复合菌制剂量与回肠绒毛高度呈显著相关关系,且0.1%复合菌制剂组达到最高. 肠道绒毛高度升高意味着管腔与营养物质接触面积增加,提升了肠道对营养物质的消化吸收能力,反过来良好的营养供给促使消化器官及肠道形态发育更好[34]. 此外,随着复合菌制剂有益微生物群落在肠道内大量繁殖,其合成维生素、氨基酸等被小肠吸收后也能促进肠道形态的发育,然而,0.2%复合菌制剂组则显著降低了绒毛高度和黏膜厚度,表明适量复合菌制剂对麻羽肉鸡肠道形态发育有积极作用,过量则抑制其发育. 较高水平复合菌制剂进入肠道后致使复合菌数量占据绝对优势,为了自身生长和繁殖将会优先利用肠上皮细胞营养物质,使肠道吸收营养物质效率降低,进而影响其发育.
本研究中,复合菌制剂显著提高了麻羽肉鸡钙和磷的消化率,可能是复合菌通过竞争体内的营养物质、附着位点等方式抑制了病原菌在肠道内的生长和繁殖;同时,复合菌制剂通过肠道微生物影响胆汁酸的合成,维生素D则要与胆汁酸结合生成胆盐后才能被肠道吸收,维生素D能促进钙和磷的消化吸收[35]. Capcarova等[25]研究也指出屎肠球菌能显著增加肉鸡对钙的吸收.
-
免疫器官指数反映了免疫器官的发育情况,其大小代表免疫系统成熟度与免疫功能强弱,是目前利用器官质量评判机体免疫性能的常用方法之一. 胸腺是细胞免疫的中枢器官,法氏囊则是禽类特有的体液免疫器官. 本研究中,0.1%复合菌制剂组提高了麻羽肉鸡胸腺和法氏囊指数,表明适量的复合菌能促进麻羽肉鸡免疫器官的发育,可能是由于复合菌作为外源性抗原物质刺激了胸腺和法氏囊的发育. Biswas等[36]研究也指出复合菌(Bacillus coagulans,Bacillus subtilis,Saccharomyces boulardi)显著提高了肉鸡脾脏和胸腺器官指数.
免疫球蛋白是介导体液免疫的主要抗体,其水平高低可在一定程度上反映机体对疾病的抵抗能力[37]. 本研究中,随复合菌制剂量的增加,IgG质量浓度呈显著的二项式升高趋势,且0.1%复合菌制剂组最高. Salim等[33]研究也指出复合菌显著提升了肉鸡IgG质量浓度. 复合菌制剂作为非特异性免疫调节因子通过提高肠道黏膜和血清IgG质量浓度增强体液免疫. IgG是血液和细胞外液的主要抗体,可结合病毒、细菌和真菌等病原体来保护机体,进而提高免疫力和抗病力[8]. 此外,0.1%复合菌制剂组显著提高了溶菌酶质量浓度,其原因是本研究所用的革兰氏阳性菌所包含的肽聚糖、脂多糖和脂磷壁酸是免疫系统的激活剂,能促进T细胞和淋巴细胞的增殖和成熟,提高吞噬细胞、自然杀伤细胞等活性,进而刺激溶菌酶的产生[38-39]. 研究已证实益生菌可通过附着在肠上皮细胞上刺激增强肠细胞介导的黏膜免疫和完整性,发挥非免疫性保护作用直接阻断肠道致病微生物[40-42].
-
肠道微生物被认为是宿主的第二大器官,经过长期的协同进化与宿主形成了互利互惠的共生关系. 本研究中,复合菌制剂对麻羽肉鸡肠道微生物α多样性影响无统计学意义,但显著影响β多样性,表明复合菌制剂不会影响麻羽肉鸡肠道菌群种类,仅影响菌群结构,保证了麻羽肉鸡在长期进化过程中形成的菌群稳定性. 此外,聚类分析显示0.05%和0.1%复合菌制剂组的肠道菌群距离较近甚至部分交叉,说明两者对菌群结构的影响相近.
肠道菌群组成上来看,所有处理的厚壁菌、拟杆菌和变形杆菌门的相对丰度共占麻羽肉鸡肠道菌群的90%以上,这与前人在蛋鸡上的研究结果类似[43],其中,0.1%复合菌制剂组的拟杆菌门相对丰度最高,而0.05%和0.1%复合菌制剂组的变形杆菌门相对丰度较低. 拟杆菌对消化复杂碳水化合物、免疫系统发育和维持肠道微生态平衡具有积极作用,表明麻羽肉鸡消化、免疫性能和肠道菌群稳定性的提高得益于适量复合菌制剂的添加引起有益拟杆菌的增殖. 变形杆菌包含多种致病菌,通过其黏附性和侵袭性改变肠道菌群,影响宿主免疫功能,诱导炎症反应产生炎症性肠炎,表明适量复合菌在一定程度上可通过抑制有害变形杆菌的增殖来预防肠道炎症. 此外,0.2%复合菌制剂组显著提高了Verrucomicrobia和Paraprevotella的相对丰度. Verrucomicrobia主要定植于胃肠道外黏液层,促进外黏蛋白层更新,增强肠上皮细胞完整性和相关黏膜免疫反应[44],并且还可利用胃肠道黏蛋白发酵产生乙酸盐、丙酸盐和乙醇,调节宿主免疫反应[45]. Paraprevotella能促进蛋白质和非纤维素多糖物质在肠道内的消化和发酵,而实际上0.1%复合菌制剂组的麻羽肉鸡表现出的生长和免疫性能更好,表明高水平复合菌制剂对单个菌的影响作用并不一定能在表型上体现出来,反而受到抑制,具体机制有待于进一步研究. Faecalibacterium可产生组蛋白去乙酰化酶抑制丁酸盐生成,丁酸盐作为能量来源,不仅具有调节肠道菌群、维持体液和电解质平衡等多种生理功能,还能抑制炎症因子的释放,保护肠黏膜屏障[46]. 本研究中,复合菌制剂显著降低Feacalibacterium的相对丰度,表明复合菌制剂能通过降低Feacalibacterium相对丰度来提高肠道丁酸盐含量,这与复合菌制剂不同程度地为麻羽肉鸡带来的益处是一致的.
3.1. 复合菌制剂对麻羽肉鸡生长性能、血清生化指标的影响
3.2. 复合菌制剂对麻羽肉鸡消化性能的影响
3.3. 复合菌制剂对麻羽肉鸡免疫性能的影响
3.4. 复合菌制剂对麻羽肉鸡肠道微生物的影响
-
在麻羽肉鸡饲粮中添加不同水平复合菌制剂可不同程度地改善其血清代谢、生长、消化和免疫性能,并对肠道菌群结构产生影响,其中0.1%为适宜添加量.






 DownLoad:
DownLoad: