-
开放科学(资源服务)标识码(OSID):

-
脂肪酸是脂质的主要成分,不仅参与生物体的生长发育[1]和营养代谢,还在生殖器官与生殖细胞的发生、发育和成熟过程中发挥调节作用. 对于哺乳动物而言,不饱和脂肪酸(Unsaturated Fatty Acid,UFA)中的前列腺素对雄性或雌性的生殖功能及生殖能力具有积极促进作用[2]. 崔亚利等[3]研究表明,在小鼠饲料中添加共轭亚油酸可以显著增加其发情期次级卵泡数量,提高胚胎数和产仔数[3]. 此外,雄性小鼠的生殖障碍与摄入的n-6/n-3多不饱和脂肪酸比值密切相关[4]. 对线虫动物的研究发现,减少n-6多不饱和脂肪酸可以使秀丽隐杆线虫(Caenorhabditis elegans)卵母细胞的大小缩小为野生型的20%,并导致后续胚胎发育和卵的孵化出现明显的不良状况[5, 6]. 在节肢动物中,褐贻贝(Pernaperna)的生殖状态与肉豆蔻酸、棕榈油酸、油酸、异油酸以及花生四烯酸等脂肪酸关系密切[7]. 此外,高不饱和脂肪酸花生四烯酸、二十碳五烯酸和二十二碳六烯酸能够显著促进甲壳类动物(如虾、蟹)的卵巢发育,并提高后代的存活率,而不饱和脂肪酸之间的相对含量比值则会影响虾和蟹的生殖性能[8-10]. 亚油酸对罗氏沼虾(Macrobrachiumrosenbergii)的卵巢成熟和繁殖力具有促进作用[11]. 当大型溞(Daphnia magna)体内16碳单不饱和脂肪酸的相对含量最多时,其产卵量和孵化率显著增加12]. 昆虫方面的研究表明,给蠋蝽(Arma chinensis)添食饱和脂肪酸会增加其后代中雌性个体的数量,而添食单不饱和脂肪酸则有利于其卵黄原蛋白的形成和生殖力的提高[13]. 此外,添食单不饱和脂肪酸能显著缩短龟纹瓢虫(Propylaea japonica)产卵潜伏期,增加产卵总数[14]. 改变饲料中的脂质浓度和ω-6/ω-3多不饱和脂肪酸比值会导致蜜蜂(Apis mellifera)产卵量的改变[15]. 另外,给昆士兰果蝇(Bactroceratryoni)幼虫添食含有棕榈油酸的小麦胚芽油可以提升其产卵量[16]. Ling等[17]的研究结果表明,通过基因操作阻止埃及伊蚊(Aedes aegypti)卵巢积累脂质,会导致卵巢发育停滞,初级滤泡消失. Gutierrez等[18]研究发现,在果蝇(Drosophila melanogaster)中,通过敲除脂肪酸ω-羟化酶基因,其卵母细胞数量减少了50%. 上述研究结果均强调了脂质与昆虫雌性生殖之间的密切关系.
作为鳞翅目昆虫之一,家蚕在丝绸生产和科学研究中有着广泛应用. 家蚕的生殖调控对蚕种繁育和害虫防治具有重要意义. 已有研究表明,家蚕5龄幼虫脂肪体的增长速度明显大于其质量的增长速度,其中雌蚕的脂肪体增长明显高于雄蚕,但蛹化后,雌蛹体内脂肪体的减少速度显著高于雄蛹,这一现象表明脂肪体是蛹期卵巢生长发育的重要营养来源[19]. 进一步研究发现,在给蚕蛹注射脂质转运颗粒抗体以抑制脂质转运后,成虫卵巢中的卵质量降低,数量减少[20],这说明脂质在家蚕雌蛹的生殖发育中具有重要作用. 目前,关于蚕蛹脂肪酸的研究主要集中在蛹油的开发和利用方面[21-27],而生殖方面有关脂肪酸的分析研究相对较少. 本研究分析了不同发育时期的雌蛹蛹体和卵巢中的脂肪酸,结果可为深入研究脂肪酸在家蚕和其他昆虫雌性生殖中的作用机制提供重要线索.
全文HTML
-
在大造蚕5龄幼虫早期,根据石渡氏腺选留雌性个体,常规常湿条件下桑叶饲养,然后选取蛹化时间相差2 h以内、蛹体大小相似且健康的雌性个体作供试个体.
-
饲养温度24 ℃~25 ℃,湿度75%~80%,选取在此条件下蛹化时间相差2 h内的雌蛹个体,从蛹化之时起,每隔24 h解剖观察1次卵巢,每个时期观察4~6个个体,至羽化前共观察到9个发育时间的卵巢形态.
卵巢组织的解剖在磷酸缓冲液(PBS)(pH=7.4)中进行,清除其它杂质后用PBS洗涤2~3次备用.
-
将刚刚摘取的卵巢于液氮中研磨,用Trizol法提取总RNA,随后用ezDNase与样品RNA在37 ℃孵育2 min,去除基因组DNA,再以oligo dT寡核苷酸为引物,与M-MLV逆转录酶(Promega)一起,参照Takara反转录试剂盒说明书合成cDNA. qRT-PCR分析用SYBR Green (T-hermofisher)荧光染料标记,以BmTIF4基因为内参,反应体系为:2xSYBR Green qRT-PCR mix 10 μL;cDNA 2 μL;引物各0.4 μL;ddH2O 7.2 μL,反应在ABI Prism 7000 Sequence Detection System (Applied Biosystems)上完成,反应条件为:① 95 ℃ 30 s;95 ℃ 5 s;60 ℃ 30 s;② 95 ℃ 15 s;60 ℃ 1 min;95 ℃ 15 s,每个样本设置3个生物学重复,相对表达量用2-ΔΔCt法计算. 利用SPSS软件中的ANOVA法进行方差分析,并选用多重比较进行显著性差异分析,其中p<0.05表示样本间差异具有统计学意义. 基因BmEP80的正向引物为AAACGGCGTCCTACAACCCTT,反向引物为TCTTGAGCGGGTGGTGGACT,基因BmA. L12的正向引物为GTGTAATGGGACAGGT,反向引物为TAGTATGGAGAACCGC.
-
将摘除卵巢组织的蛹体捣碎研磨,充分混匀,使其总质量保持在5 g以上,用于蛹体脂肪酸分析;将卵巢捣碎研磨,充分混匀,蛹化1~3 d的卵巢使其总质量保持在0.5 g以上,蛹化4~6 d的卵巢使其总质量保持在1 g以上,蛹化7~9 d的卵巢使其总质量保持在3 g以上,用于卵巢脂肪酸分析.
-
精确称取0.500 0 g样品,研磨均匀后置于10 mL离心管中,每组样品进行3次生物学重复. 向匀浆中加入5 mL氯仿-甲醇混合液,高速振荡混匀1 min,然后50 ℃超声提取1.5 h. 超声结束后,4 000 rpm离心2 min,将上清液转移到新离心管,重复以上提取步骤一次,并混合两次提取液. 然后向提取液中加入2 mL饱和NaCl,振荡混匀30 s,4 000 rpm离心10 min. 将有机相转移到新离心管,加入0.5 g无水硫酸钠干燥. 4 000 rpm离心10 min后,将上清液转移至新离心管,50 ℃氮吹干燥,得到粗脂肪. 随后向粗脂肪中加入5 mL正己烷和3 mL氢氧化钠溶液,振荡混匀,放入烘箱中60 ℃甲酯化30 min. 4 000 rpm离心10 min,取上清1 mL过0.22 μm有机滤膜,最后加入进样瓶中进行气相色谱检验.
-
Agilent GC 7890气相色谱仪,FID检测器,DB-FFAP毛细管色谱柱(60 m*0.25 mm*0.5 mm). 进样量:1 μL;进样口温度:250 ℃;后检测器温度:280 ℃;柱温箱保持180 ℃恒温. 分流进样,分流比为50∶1,流速:1 mL/min. 流量比:氢气∶空气∶氮气为40∶400∶40.
-
从气相色谱检验中得到了不同脂肪酸的峰面积,并以此计算出每种脂肪酸在总峰面积中的占比. 对3次生物学重复进行均值计算. 最后得到的结果即为脂肪酸的相对含量.
1.1. 供试材料
1.2. 卵巢解剖
1.3. RNA提取、cDNA合成以及实时荧光定量PCR(qRT-PCR)分析
1.4. 用于脂肪酸检测样品采集
1.5. 样品处理
1.6. 样品检测
1.7. 数据处理
-
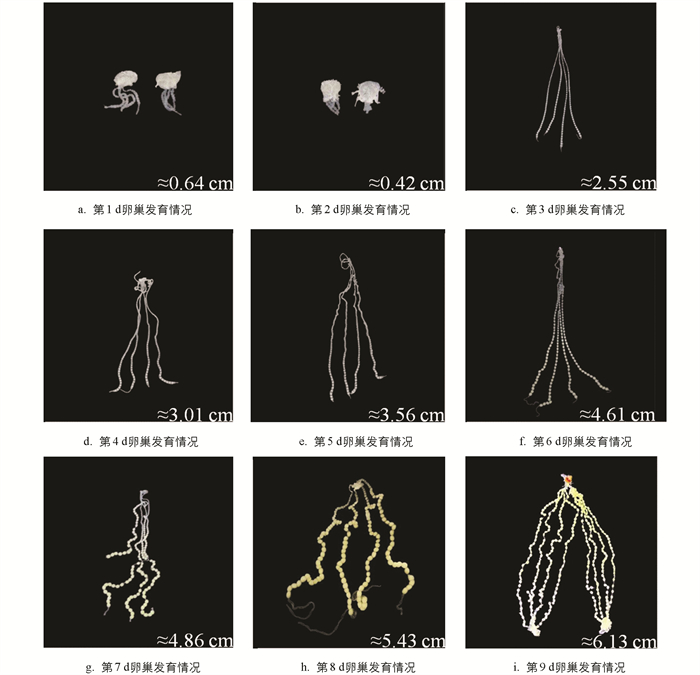
家蚕雌蛹有左右两侧对称卵巢,每侧卵巢有4根卵巢管. 家蚕雌蛹不同发育时期的卵巢形态如图 1所示,仅在蛹化第1,2 d和蛹化第9 d(羽化前1 d)同时显示了左右两侧的卵巢,其余时间均显示一侧卵巢.
在蛹化当天(图 1a),可以观察到卵巢管突破卵巢被膜,随着发育的进行,卵巢管不断在体腔内延伸,到蛹化第3 d,卵巢管长度显著增加(图 1a-1c),并向体腔延长扩展. 在蛹化初期,卵管细,管内卵小,且呈透明状,从蛹化第4 d开始,可以观察到卵巢管增粗,到了蛹化第6 d,卵巢管最远端的卵内出现明显的营养物质积累,伴随着卵体积的增大,卵色由白色转变为淡黄色(图 1d-1f). 蛹化第7 d,卵巢管进一步扩张并充满体腔,卵巢管中正在积累营养物质的卵以及营养物质积累完成的卵的数量由远及近显著增加(图 1g). 到了蛹化第8 d,卵巢管内的大部分卵已经完成了营养物质的积累,远端的少部分卵形成卵壳,形状也由圆形变为椭圆形,卵色变淡,卵间桥连消失,彼此分离,卵子发育成熟(图 1h). 蛹化第9 d,卵巢几乎占据了整个体腔,卵巢管内的大部分卵发育成熟,等待排出体外(图 1i).
-
卵巢发育和卵子发生是一个连续的过程. 在蛹期,卵巢发育的同时,卵管内的卵子也在迅速发育,直到羽化时,卵巢内的所有卵子均发育成熟. 卵子发生可以分为6个阶段:卵黄生成前期、卵黄生成期、卵黄生成末期、卵壳生成前期、卵壳生成期和卵壳生成末期[27]. 通过对卵巢形态和卵管内卵子的连续观察,结合蛹期的发育过程,将蛹化后的4个时期作为卵巢发育和卵子发生的代表性时期,即:蛹化第1 d(卵巢管突破卵巢被膜,管内卵子依稀可见)、蛹化第3 d(卵巢管快速延伸增长,管内卵子透明且清晰可见)、蛹化第6 d(卵巢管最远端卵子内有明显的营养物质积累,体积增大,颜色由白色转为淡黄色)和蛹化第9 d(除近端的少数卵外,卵管内的大部分卵子成熟,形成卵壳,形状由圆形变成椭圆形,卵间桥连消失,彼此分离并移向输卵管).
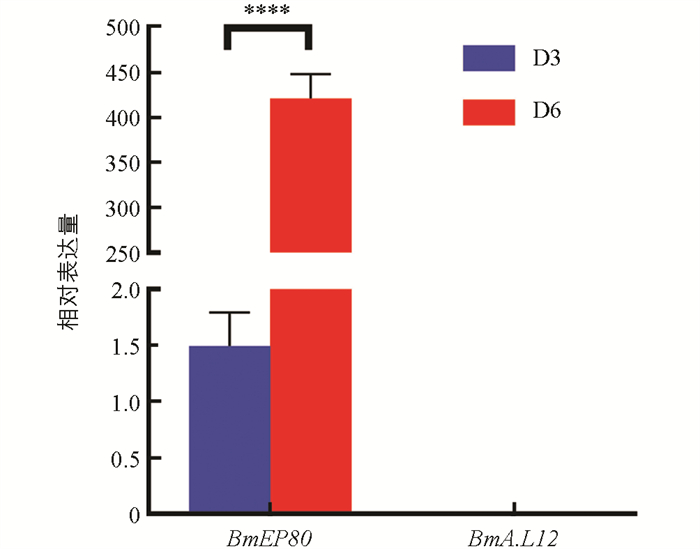
在卵管内,卵子按线性顺序依次发育,相邻卵子之间的发育时间间隔为2~2.5 h,最先发育和成熟的是最远端的卵子[27]. 然而,这种顺序发育的特点可能导致上述时期在卵子发生方面的代表性不足. 为此,使用qRT-PCR分析了蛹化第3 d和蛹化第6 d卵巢中卵黄膜蛋白编码基因BmEP80 (卵黄生成期标志基因)和绒毛膜B类蛋白L12基因BmA.L12 (卵壳生成期标志基因)的表达情况. 结果如图 2所示,卵黄生成期标志基因BmEP80在蛹化第3 d微量表达,在蛹化第6 d有高量表达,两个时期的基因表达差异具有统计学意义. 而卵壳生成标志基因BmA. L12在两个时期均未检测到表达. 因此,可以得出以下结论:①蛹化第3 d,卵子发育处于卵黄生成的萌发阶段;②蛹化第6 d,卵子发育正处于卵黄大量生成阶段,卵壳形成尚未开始. 这两个时期在卵子发生上存在明显差异,可以作为卵子发生阶段的代表性时期. 故蛹化后卵子发生阶段的4个代表性时期为:蛹化第1 d,卵子发育启动;蛹化第3 d,卵黄生成开始;蛹化第6 d,卵黄大量生成;蛹化第9 d,卵子成熟,卵壳生成完毕.
-
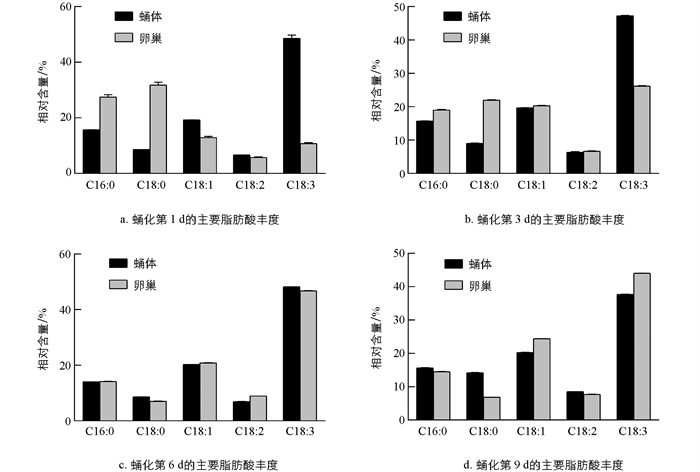
利用高效气相色谱(GC)从蛹体的粗脂肪中搜索C4至C24共37种脂肪酸,结果检测到了12种脂肪酸,分别是肉豆蔻酸、十五碳烯酸、棕榈酸、棕榈油酸、珍珠酸、银杏酸、硬脂酸、油酸、亚油酸、α-亚麻酸、花生酸、花生三烯酸. 在蛹体中,α-亚麻酸含量最丰富,丰度最高,为37.687%~48.640%,油酸次之,丰度为19.262%~20.280%,后面依次是棕榈酸、硬脂酸和亚油酸,丰度分别为14.085%~15.716%,8.613%~14.199%,6.349%~8.501%,这些脂肪酸总丰度在96%以上,属于蛹体的主要脂肪酸,其余7种脂肪酸总丰度不超过4%,属于微量脂肪酸(表 1,表 2).
蛹化第1 d到第6 d,蛹体中各主要脂肪酸的丰度变化较小. 然而,从蛹化第6 d到第9 d,可以观察到α-亚麻酸丰度明显下降,从48.207%降至37.687%,下降幅度为10.520%;相反,硬脂酸的丰度显著增加,从8.613%上升至14.199%,增长幅度为5.586%. 在此期间,亚油酸的丰度略微升高,从6.948%上升至8.501%,增长幅度为1.553%. 其它两种主要脂肪酸的丰度变化幅度较小(表 1). 主要脂肪酸的总丰度在蛹化第1 d至第6 d变化较小,而在第9 d出现明显下降(表 1). 7种微量脂肪酸的丰度变化方向各不相同,但总体随发育的进行而增加,这与主要脂肪酸的变化趋势不同. 值得注意的是,微量脂肪酸在蛹化第1 d至第6 d的变化幅度明显小于第6 d到第9 d的变化幅度,这与主要脂肪酸的变化特征相似(表 2).
-
在卵巢中,同样检测到了12种脂肪酸,其中α-亚麻酸、油酸、棕榈酸、硬脂酸和亚油酸的丰度均超过5%,属于主要脂肪酸,其总丰度为88%~98%(表 3);剩余7种脂肪酸是微量脂肪酸,其总丰度在蛹化第1 d以外的其它3个时期都低于6%(表 4).
与蛹体不同,卵巢中大多数主要脂肪酸在整个发育过程中均发生了明显变化,蛹化第1 d至第6 d的变化幅度大于第6 d至第9 d的变化幅度(表 3,表 4). 总体而言,脂肪酸变化的趋势如下:随着发育的进行,不饱和脂肪酸的丰度增加,饱和脂肪酸的丰度下降(表 3). 其中,油酸的丰度随发育不断上升,而硬脂酸的丰度随发育不断下降;α-亚麻酸和亚油酸的丰度从蛹化第1 d至第6 d持续上升,然后略微下降;棕榈酸的丰度在蛹化第1 d至第6 d先下降,后略微升高(表 3). 整个过程变化幅度最大的是α-亚麻酸,其丰度从10.760%上升至46.802%,增长幅度为36.042%,其次是硬脂酸,其丰度从31.811%下降至6.835%,下降幅度为24.976%;棕榈酸的丰度从27.469%下降至14.171%,下降幅度为13.298%;油酸的丰度从12.937%上升至24.420%,增长幅度为11.483%;亚油酸的丰度由5.758%上升至8.978%,增长幅度为3.220%,变化幅度最小(表 3). 卵巢中主要脂肪酸的总丰度在蛹化第1 d至第6 d随发育不断升高,但增长速度逐渐减缓,在第6 d至第9 d变化甚微. 卵巢主要脂肪酸的总丰度呈上升趋势.
卵巢微量脂肪酸在整个发育过程中丰度变化显著,且蛹化第1 d至第6 d的变化幅度大于蛹化第6 d至第9 d的变化幅度(表 4). 与主要脂肪酸的变化趋势不同的是,卵巢中微量脂肪酸的总丰度随发育逐渐下降.
-
通过比较雌蛹蛹体和卵巢中的主要脂肪酸丰度可以发现,在蛹化初期,卵巢主要脂肪酸中的不饱和脂肪酸丰度低于蛹体,特别是α-亚麻酸和油酸,二者在卵巢中的丰度明显低于蛹在体中的丰度,而卵巢中的饱和脂肪酸(硬脂酸和棕榈酸)的丰度明显高于蛹体(图 3). 随着发育的进行,卵巢中各主要脂肪酸的丰度与蛹体中主要脂肪酸的丰度之间的差距逐渐缩小,直到趋于一致,但在发育后期又出现了差异. 具体而言,α-亚麻酸丰度差异在蛹化第1 d为37.880%,第3 d为21.095%,第6 d为1.045%,第9 d为6.366%;硬脂酸丰度差异在蛹化第1 d为23.191%,第3 d为12.958%,第6 d为1.520%,第9 d为7.364%;棕榈酸丰度差异在蛹化第1 d为11.753%,第3 d为3.330%,第6 d为0.086%,第9 d为1.106%;油酸丰度差异在蛹化第1 d为6.325%,第3 d为0.630%,第6 d为0.619%,第9 d为4.158%;亚油酸丰度差异在蛹化第1 d为0.924%,第3 d为0.352%,第6 d为2.030%,第9 d为0.765%(表 5). 蛹化第6 d,卵巢和蛹体中的α-亚麻酸、油酸、硬脂酸和棕榈酸丰度,差异最小,但到了蛹化第9 d,卵巢和蛹体中相应脂肪酸的差异略微增大. 与其它主要脂肪酸相比,亚油酸丰度变化稍有不同:蛹化第3 d,它在蛹体和卵巢中的丰度差异最小,而在蛹化第6 d,丰度差异最大(图 3b,3c). 微量脂肪酸在蛹体和卵巢之间的丰度差异变化趋势与主要脂肪酸相似,即在蛹化初期差异最大,随发育逐渐缩小. 除个别脂肪酸(如棕榈油酸)在卵巢和蛹体中的丰度差异在蛹化第9 d最小外,其余微量脂肪酸在蛹化第6 d差异最小,第9 d差异略有增加(表 2,表 4).
2.1. 家蚕蛹期卵巢形态学观察
2.2. 雌蛹发育过程中卵巢发育和卵子发生阶段的代表性时期
2.3. 蛹发育过程中雌蛹体脂肪酸丰度
2.4. 雌蛹发育过程中卵巢脂肪酸丰度
2.5. 蛹发育过程中雌体卵巢脂肪酸丰度差异
-
家蚕蛹期的雌性生殖发育包括卵子发生和卵巢发育. 卵子发生随卵巢发育而启动[28]. 在发育过程中,卵巢会出现明显的形态变化. 然而,由于卵巢管内排列的众多卵子具有不同步的连续发育时间特性,仅依靠卵巢形态观察来区分雌性生殖发育阶段的代表性时期可靠性较低. 为了解决这一问题,研究采用qRT-PCR分析了卵巢卵黄生成标志基因BmEP80和卵壳生成标志基因BmA.L12,在蛹化第3 d(卵管快速伸长期)和蛹化第6 d(初见卵内营养物质积累期)的表达水平. 研究结果显示,蛹化第3 d卵子发育处于卵黄生成的萌发阶段,而蛹化第6 d卵子发育正值卵黄大量生成和卵壳形成前期. 这两个时期在卵子发生方面表现出明显差异,因此具有代表性,可以用来区分雌性生殖发育阶段.
研究检测了不同发育时期雌蛹体内脂肪酸,结果显示,在所有时期均检测到了12种脂肪酸的存在. 其中,棕榈酸、硬脂酸、油酸、亚油酸、α-亚麻酸的丰度较高,被认为是主要脂肪酸,这一结果与之前的报道一致[20-26]. 不同的是,α-亚麻酸是蛹体中丰度最高的脂肪酸,与大多数雌雄混合分析认为油酸是家蚕蛹体含量最高的脂肪酸不同,可能是α-亚麻酸含量在雌蛹比在雄蛹高的缘故[29-30]. 此外,还检测到了7种微量脂肪酸,包括肉豆蔻酸、棕榈油酸、花生酸、花生三烯酸、十五碳烯酸、十七烷酸和银杏酸. 然而,研究未检测到蔡沙等[25]和张雨丽等[22]发现的十三碳酸、月桂酸(十二烷酸)和十九酸.
雌蛹脂质含量随发育快速减少[19, 31]. 结果显示,在蛹化第1 d至第6 d,雌蛹蛹体中各主要脂肪酸的丰度变化较小且相对稳定. 这表明,在这个发育阶段,主要脂肪酸被消耗利用的速度与总脂质下降的速度相似,并且各主要脂肪酸利用均衡. 蛹化第6 d至第9 d,蛹体内主要脂肪酸的总丰度下降,这可能是丰度最高的α-亚麻酸显著下降所致. 这表明,在这个时期,雌蛹蛹体对α-亚麻酸有偏好利用的趋势. 与此同时,蛹体中硬脂酸和亚油酸丰度发生了明显增加,这可能源于蛹体中丰度最高的α-亚麻酸消耗较多,导致丰度相对较低的脂肪酸出现增加,这些脂肪酸在机体中消耗利用较少甚至不被利用. 此外,随着发育的进行,蛹体中微量脂肪酸的总丰度逐渐增加,尤其是在蛹化第6 d至第9 d,当主要脂肪酸的丰度出现下降时,微量脂肪酸的丰度明显上升. 这可能是由于机体利用和消耗微量脂肪酸的速度低于主要脂肪酸或总脂肪酸的速度. 本研究中雌蛹体内的5种主要脂肪酸的丰度,虽然在大小顺序上与现有的报道一致,但在具体的丰度上有些脂肪酸存在明显差异,这可能源于所用实验材料和方法不同[29].
蛹化后,卵巢随发育迅速增长. 在蛹化初期,卵巢内含有细小的卵管和少量卵. 随着卵管的快速延伸和卵内营养物质的积累,卵巢迅速增大,并最终占据了蛹体大部分的体腔. 据报道,羽化前卵巢中的脂质含量是蛹化当天卵巢中脂质含量的100多倍,并且羽化前单位卵巢质量的脂质浓度比蛹化当天单位卵巢质量的脂质浓度高75%以上,卵巢随发育聚集了大量脂质[31]. 结果显示,蛹体内的5种主要脂肪酸同样也是卵巢中的主要脂肪酸. 在整个蛹期,卵巢各主要脂肪酸的丰度随发育不断变化,不同类型的脂肪酸的变化幅度和方向各不相同. 其中,不饱和脂肪酸的丰度随发育增加,饱和脂肪酸丰度则显著下降. 在发育过程中卵巢对不同类型脂肪酸的积累速度存在差异. 卵巢更倾向于积累不饱和脂肪酸,因此α-亚麻酸和油酸丰度显著增加. 与蛹化第6 d相比,蛹化第9 d卵巢主要脂肪酸的总丰度变化微小. 然而,丰度最高的α-亚麻酸发生下降,而油酸的丰度明显升高,这表明,在这个阶段卵巢积累α-亚麻酸的速度下降、而积累油酸的速度提高,很可能与卵壳生成、卵子成熟有关. 有研究表明雌蛹卵巢脂肪酸的丰度在发育过程中变化较小且相对稳定,α-亚麻酸与硬脂酸的丰度相似,并明显低于油酸、棕榈酸和亚油酸的丰度. 这与本实验结果有很大不同[31-32]. 然而,他后来提出的家蚕早期胚胎中主要脂肪酸的丰度大小顺序与本实验结果中蛹化第9 d卵巢的主要脂肪酸丰度大小顺序完全一致[31-32].
蛹化后,蛹体的脂质逐渐减少,而卵巢中的脂质明显增加[19, 31]. 研究结果显示,蛹体中主要脂肪酸的总丰度呈现下降趋势,而卵巢中主要脂肪酸明显上升. 相反,微量脂肪酸的总丰度在蛹体中上升,在卵巢中明显下降,这表明随着发育的进行,主要脂肪酸在蛹体中大量消耗、在卵巢中大量积累,而微量脂肪酸在蛹体中的消耗较少、卵巢中的积累也较少,由此推测主要脂肪酸与蛹期雌性生殖发育的相关性大于微量脂肪酸.
昆虫卵泡在体外新合成的脂肪酸量相当于成熟卵子中脂肪酸的1%左右,而卵巢自身很少或几乎不合成脂肪酸[33-34]. 卵巢中积累的脂质主要来自蛹体转运[31]. 对比蛹体与卵巢中主要脂肪酸的丰度发现,二者在蛹化当天存在显著差异,随着发育的进行,卵巢和蛹体之间的差异逐渐缩小,卵巢中原本丰度低于蛹体的脂肪酸开始增加,而原本丰度高于蛹体的脂肪酸开始下降. 蛹化第6 d,棕榈酸、硬脂酸、油酸和α-亚麻酸的差异最小,即此时它们在卵巢的丰度与蛹体的丰度相似. 从蛹化第1 d至第6 d,蛹体中各主要脂肪酸的丰度变化较小,而这个发育时期中蛹体棕榈酸、硬脂酸、油酸、α-亚麻酸的丰度,与蛹化第6 d卵巢中相应脂肪酸的丰度接近,这种同质性表明卵巢发育和卵黄生成对蛹体的高度依赖性. 至蛹化第9 d,这4种主要脂肪酸的丰度差异略微增加,可能与卵壳生成和卵子成熟有关,同时也与成虫器官重塑即将完成有关,因为在这个阶段,蛹体中主要脂肪酸的消耗速度超过总脂质的消耗速度,而卵巢中主要脂肪酸的积累速度与总脂质的积累速度接近. 与前述4种主要脂肪酸不同,亚油酸在蛹化第3 d的差异最小,而在蛹化第6 d的差异最大,它在卵巢中的丰度显著升高并明显高于蛹体中亚油酸的丰度,出现了亚油酸的丰度高于硬脂酸的情况,这种现象一直持续到蛹化第9 d. 这与蛹体中硬脂酸丰度高于亚油酸丰度的恒定顺序不同,显示了卵巢发育卵子发生的特异性.
-
通过分析卵巢发育卵子发生特征时期蛹体-卵巢脂肪酸丰度,显示了卵巢发育、卵子发生过程中蛹体脂肪酸消耗和卵巢脂肪酸积累的特征,为进一步深入探究脂肪酸在变态昆虫(如家蚕)生殖中的作用及机制提供了线索.



 下载:
下载: