-
绝大多数蜘蛛是独居的,分布较分散,且具有攻击性和性食同类现象,它们的繁殖需要交配选择信号来发现配偶并且评估配偶的繁殖价值,决定是否接受配偶[1-3].结网型蜘蛛的视力较差,蛛网的存在可以帮助它们识别猎物、捕食者和潜在的配偶,研究表明它们更多地依靠触觉和化学信号来交流[4-6].在交配季节,雄蛛离开自己的网,寻找雌蛛进行交配.一方面,雌蛛通过释放表皮的或沉积在蛛网上的化学物质吸引雄蛛,这些化学物质可以传递不同类型的信息,比如:物种身份、年龄、性别以及生殖状态,通常还可以激发雄蛛的求偶行为[7-8].另一方面,雄蛛通过触肢上的化学感受器感知雌蛛释放的性信息素,根据交配代价和雌蛛质量的变化,进行配偶选择[9-11].雄蛛的求偶行为被认为可以进行物种识别、刺激雌性的性行为、抑制雌性交配前的捕食倾向,因此,描述雄蛛的求偶行为对于理解它的繁殖生物学是很重要的[12-15].
雌蛛的性成熟状态和交配状态对于雄蛛来说可能是繁殖价值的指示剂,直接影响雄蛛的交配投资[1].研究表明,许多无脊椎动物的雄性可以识别已交配雌性和处女雌性,对求偶强度、精液大小和相应的容量进行调整[16-21].尤其对于存在性食同类的类群,潜在的配偶也是潜在的捕食者,雄性错误靠近异种或不接受交配的雌性将面临被捕食的风险,雄蛛通过蛛丝区分处女雌蛛和已交配雌蛛已经在许多研究中得以报道[22-26],相对来说,雄蛛更倾向于选择处女雌蛛的蛛网[27-29].性选择使雄蛛把搜寻代价降到最低,提高区分雌蛛生殖状态的能力,放弃繁殖价值低的雌蛛,避免随之而来的代价和风险[22].
目前关于漏斗蛛科性信息素的研究较少,已鉴定得到信息素化学结构的仅有1种. Riechert & Singer报道了北美的一种沙漠漏斗蛛(Agelenopsis aperta),雌蛛蛛网上存在性信息素,并且雄蛛可以对雌蛛的交配状态进行选择[25]. Papke等报道了这种漏斗蛛的性信息素为8-甲基-2-壬酮,它既可以吸引雄蛛,也能引起雄蛛的求偶行为[30].本实验以刺近隅蛛为研究对象,采用动物行为学的方法探究:① 求偶及交配行为的具体历程;② 雌蛛蛛网上是否存在引起雄蛛求偶的化学物质;③ 雄蛛是否可以根据蛛网上的化学物质判断雌蛛的交配状态,揭示化学物质在漏斗蛛科性选择中的作用,为信息素的提取和鉴定提供实验依据.
HTML
-
刺近隅蛛(Aterigena aculeata)主要分布在中国华北、华中、华南和西南地区.通常在地面附近结漏斗状网,网中间有一细管伸向落叶层,5-7月成熟.于2014年6月底在重庆市缙云山采集亚成体雌蛛和雄蛛,实验室条件下饲养成熟.采回的蜘蛛单头分装在80 mL塑料瓶中,底部用蘸湿的棉花保湿,置于温度为28 ℃的培养箱(RXZ-300A)中培养,光照时间14 h.蜘蛛用黄粉虫幼虫(Tenebrio molitor)和黑腹果蝇(Drosophila melanogaster)喂养,记录每头蜘蛛的成熟时间.
-
随机选取30对至少成熟两周的雌雄蛛进行交配.实验前1 d,将雌蛛放在实验盒(9×9×15 cm3)中结网,随后引入雄蛛,用DV(SONY-PJ820E)拍摄记录求偶及交配行为,每对观察2 h或直到交配结束.交配结束后将雌雄蛛分开.记录以下指标:求偶、交配行为模式;求偶延迟时间;求偶持续时间;交配持续时间;触肢插入次数、插入时间.
-
用双向选择盒探究雌蛛蛛网上是否存在信息素,能否引起雄蛛的求偶行为.双向选择盒包括中间的释放盒和两侧的选择盒(9×9×3.5 cm3),中间用小的圆形塑料盖连接.实验前,将装置用清水洗净,再用75%的酒精清洗,晾干.将处女雌蛛(n=32) 放入一侧选择盒结网,24 h后移出雌蛛,留下空网.连接好实验装置,中间为释放盒,一侧选择盒是雌蛛蛛网,另一侧是空白对照,每两套装置的位置交叉摆放.随机选取成熟雄蛛放入释放盒,记录雄蛛选择的位置.在重复实验之前,将装置洗净,晾干.
-
通过4种不同状态的蛛网探究雄蛛对蛛网的区分能力.分别将亚成体雌蛛(n=15),处女雌蛛(n=20),已交配雌蛛(n=20) 和雄蛛(n=20) 放入实验盒(9×9×15 cm3)中结网24 h,之后移出蜘蛛,留下空网.随机选取成熟雄蛛引入蛛网,用DV拍摄记录求偶行为.记录雄蛛求偶延迟时间,每头蜘蛛只用1次.
-
用卡方检验(chi-square)来确定双向选择中雄蛛选择的观察值和预期值;不同状态的蛛网对雄蛛的吸引力实验中,采用单因素方差分析(One-way ANOVA)进行比较,最小显著差异法(LSD)进行事后多重比较检验.数据表示为平均值±标准误,数据处理在SPSS 22.0中完成.
1.1. 研究材料
1.2. 研究方法
1.2.1. 求偶和交配行为
1.2.2. 蛛网对雄蛛的吸引力
1.2.3. 不同状态蛛网对雄蛛的吸引力
1.2.4. 数据处理
-
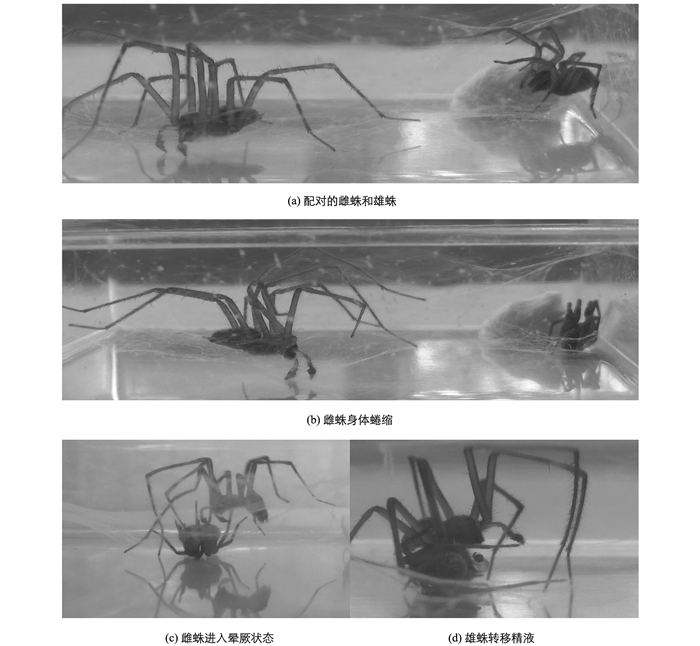
将刺近隅蛛雄蛛引入雌蛛所在实验盒,通常经过一段静止时间后,雄蛛开始求偶,身体在雌蛛蛛网上沿垂直方向上下振动,并逐渐靠近雌蛛.若雌蛛蜷缩不动,雄蛛立马冲上前去咬雌蛛的步足,雌蛛随即进入僵直状态.雄蛛将雌蛛拖至管口处,调整雌蛛位置利于触肢的插入,开始交配(如图 1).交配期间雌蛛一直处于僵直状态,雄蛛触肢在抽捋2~3次后插入,触肢器插入后血囊迅速膨大,再快速缩小,之后停顿1~5 s,重复“抽捋—插入—膨大—缩小—抽出”的过程.每侧触肢平均插入15次,平均插入时间为1 min 27 s.交配结束后雄蛛离开,雌蛛很快苏醒.交配成功率为83.33%,求偶延迟时间、求偶持续时间和交配持续时间分别平均为9 min,11 s和6 min.雌、雄蛛都可进行二次交配.雄蛛在交配前和交配后有毁网行为,把雌蛛蛛网的丝线切断,破坏网的结构,使网团在一起,有的也存在结网行为.
-
雄蛛引入释放盒后通常在静止一段时间后做出选择.实验结果如图 2所示,相比于空白对照组(10/32),雄蛛更多地选择处女雌蛛蛛网(20/32),且两者有显著性差异(x2=15.25,p<0.05).大多数雄蛛在蛛网上表现明显的求偶行为.
-
雄蛛在不同状态的蛛网上的求偶延迟时间见表 1,图 3.雄蛛在处女雌蛛蛛网上的求偶延迟时间最短(10.21±2.04 min),明显短于在其他3组蛛网上的延迟时间(p<0.05).雄蛛在已交配雌蛛蛛网上也表现求偶行为(86.11±4.08 min),但求偶延迟时间长于处女雌蛛蛛网,与雄蛛蛛网和亚成体雌蛛蛛网差异有统计学意义(p<0.05).雄蛛蛛网和亚成体雌蛛蛛网不能引起雄蛛的求偶行为.
2.1. 求偶和交配行为
2.2. 蛛网对雄蛛的吸引力
2.3. 不同状态的蛛网对雄蛛吸引力
-
刺近隅蛛的求偶模式为身体沿垂直方向上下抖动,随着雌蛛进入晕厥状态,雄蛛将一侧触肢插入雌蛛的交配孔,交配开始,重复“抽捋—插入—膨大—缩小—抽出”的过程,交配结束后,雄蛛离开,雌蛛逐渐苏醒.伴有交配前和交配后的毁网行为.
雌蛛是贪婪的捕食者,雄蛛接近雌蛛时,必须展现有效的求偶信号抑制雌蛛的捕食倾向,这就需要雄蛛在吸引雌蛛的同时让雌蛛能够辨别自己是同种个体.为了获得成功的交配,雄蛛在接近雌蛛时采取了一定的交配策略.首先,雄蛛在交配前展现的一系列求偶振动行为降低了被雌蛛捕食的风险;第二,交配前和交配后的毁网行为,可能减少了蛛网上性信息素的挥发,从而减少了其他雄蛛接近蛛网干扰其交配的几率[31].一些蜘蛛的交配过程也有类似的现象,如三角皿蛛(Linyphia triangularis),雄蛛到达未交配雌蛛的蛛网后开始切断蛛网的丝线,然后把大部分丝卷成一个小球[32];澳大利亚红背蜘蛛(Latrodectus hasseltii)雄蛛在求偶时会拆除雌蛛的网,使雌蛛在交配后重新结网[26, 33];另外,随着雄蛛求偶行为的进行,雌蛛身体蜷缩不动,继而进入晕厥状态,并一直持续到交配结束,也是一种交配策略.
性食同类现象存在于蜘蛛的许多类群中,雄蛛在交配前、交配中或交配后都可能被雌蛛捕食,若雌蛛进入性晕厥状态,整个交配状态由雄蛛主导和控制,可以确保安全地完成交配[34-37]. Becker等用实验验证沙漠漏斗蛛(Agelenopsis aperta)雄蛛引起雌蛛进入僵直状态的机制,发现雄蛛的身体匀浆可以使雌蛛产生晕厥行为,证明雄蛛产生的信息素使雌蛛进入僵直状态[36].模拟Hololena curta雄蛛的振动会降低雌蛛的攻击性,但不能引导雌性进入静止甚至僵直的状态,说明还需要化学的或接触的信号[37].但是到目前为止,还没有得到任何一种漏斗蛛雄蛛信息素的结构,刺近隅蛛引起雌蛛晕厥的机制还需要进一步的研究.
在双向选择实验中,雄蛛选择处女雌蛛蛛网的数量显著多于选择空白对照的数量,而且在蛛网上出现求偶行为,表明处女雌蛛的蛛网上存在信息素吸引雄蛛并引起雄蛛的求偶行为.雄蛛在4种不同状态的蛛网上的求偶延迟时间不同,说明刺近隅蛛雄蛛有能力根据蛛网上的信息素判断雌蛛的性成熟状态.已经达到性成熟的雌蛛,为了获得精子,避免没有交配的风险,会在蛛丝上分泌信息素吸引雄蛛;而未成熟的雌蛛因为缺少受精的直接收益,雄蛛的求偶反而会带来高捕食率的风险,所以不被期望得到雄蛛的投资;已交配雌蛛为了获得二次交配的好处,依然会吸引雄蛛,但为了避免二次交配的风险,会降低对雄蛛的吸引力.雄蛛之所以更偏爱处女雌蛛,是因为对于有多次交配的蜘蛛来说,与处女雌蛛交配可以得到更高的受精率,而处女雌蛛比已交配雌蛛更容易接受求偶的雄蛛,减少了交配前的性食同类[37-38].至于刺近隅蛛吸引雄蛛的信息素成分以及雌蛛在交配前后信息素发生了怎样的变化是在接下来的研究中亟待解决的问题.








 DownLoad:
DownLoad: