-
卵母细胞体外成熟技术可为研究植入前胚胎发育和体细胞克隆等提供实验材料.已有研究表明,组蛋白修饰参与了哺乳动物卵母细胞成熟过程,并且其表达模式在不同物种卵母细胞成熟过程中存在差异.组蛋白甲基化是调控真核生物细胞内转录状态的重要修饰之一,其生物学功能主要与转录调控、基因组完整性及表观遗传继承等有关.组蛋白甲基化主要发生在H3/H4组蛋白尾部氨基酸残基位点上,共分为单甲基化、双甲基化和三甲基化.不同位点组蛋白H3氨基酸位点的甲基化可对转录产生激活或者抑制作用.组蛋白H3第4位赖氨酸(H3K4) 甲基化通常富集于转录起始位点[1],是转录激活的标志表观修饰之一;组蛋白H3第9位赖氨酸(H3K9) 甲基化是经典的基因沉默修饰[2],通常与异染色质的形成有关.
Kageyama等[3]研究表明,在小鼠卵母细胞中H3K4和H3K9甲基化的表达随着卵泡发育而逐渐增强,并且在生发泡期中表达信号最强.在牛的卵母细胞体外成熟过程中,组蛋白甲基化呈现出不断变化的状态.在猪的初级、次级和早期窦卵泡附近均表现出显著的组蛋白甲基化修饰,卵丘附近呈现出H3K4强甲基化状态.这些研究说明,组蛋白甲基化参与了哺乳动物卵子发生过程的基因转录调控作用.此外,组蛋白甲基化也是影响哺乳动物克隆胚胎发育的重要因素. H3K4甲基化参与调控小鼠供体细胞核中多能性基因,如Sox2,Oct4等基因的表达,进而影响供体细胞核的重编程效率[4].通过在小鼠胚胎干细胞中短时表达去甲基化转移酶基因Jmjd2b,可调节克隆胚胎中H3K9三甲基化表达水平,提高克隆胚胎体外发育效率[5].
H3K4和H3K9甲基化在调控哺乳动物卵母细胞和克隆胚胎发育中起到重要的作用,目前有关H3K4三甲基化(H3K4me3) 和H3K9三甲基化(H3K9me3) 在体外成熟猪卵母细胞和克隆胚胎中的表达模式还鲜有报道.本研究通过观察H3K4me3和H3K9me3在猪卵母细胞和体细胞克隆胚胎中的表达模式,将有助于了解体外成熟卵母细胞和克隆胚胎中基因组表达动态变化,并且为改善猪卵母细胞成熟质量,提高克隆胚胎发育效率奠定基础.
HTML
-
猪卵母细胞体外成熟液(Porcine maturation medium,PM);猪胚胎体外培养液(Porcine zygote medium-3,PZM-3);一抗:组蛋白H3第4位赖氨酸三甲基化位点(H3K4me3) 兔源多克隆抗体(Abcam,1:100),组蛋白H3第9位赖氨酸三甲基化位点(H3K9me3) 兔源多克隆抗体(Abcam,1:200);二抗:Dylight 488标记的山羊抗兔IgG(Thermo,1:400);固定液:4%多聚甲醛的PBS溶液;清洗液:含0.01% Triton X-100和0.3% BSA的PBS溶液;阻断液:含0.3% BSA和0.1 mol/L甘氨酸的PBS溶液.
CO2培养箱(Thermo公司);超净工作台(AIRTECH公司);体视显微镜(NIKON公司);显微操作仪(NIKON公司);LSM 510 meta激光扫描共聚焦显微镜(Leica公司);体视镜热台、可调式恒温热台(Thermo公司);融合仪(BLS,CF-150B);融合槽(BTX,450-1).
-
猪卵巢来源于广西南宁市本地屠宰场,保存在装有30 ℃双抗生理盐水的保温壶中于2~3 h内运回实验室.卵巢用75%的乙醇溶液清洗一遍,时间不超过15 s,随后用加热至34 ℃的DPBS清洗3遍,于39 ℃水浴锅中待用.然后用带有12号针头的10 mL注射器抽取直径为3~6 mm的卵泡,卵泡液打入10 mL玻璃圆底试管中,39 ℃保温,自然沉淀15 min.去除三分之二上清,将剩余卵泡液打入60 mm玻璃平皿中,用洗卵液混匀,在体式显微镜下挑选有3层以上卵丘细胞的卵丘-卵母细胞复合物(Cumulus oocyte complexes,COCs),COCs用洗卵液清洗3遍后每30~50个放入盖有矿物油并做有150 μL的PM微滴,前22 h置于含有激素的PM中,后20 h移入不含激素的PM中继续培养,培养箱条件为38 ℃,5% CO2,100%湿度.
-
剪取2个月大的猪耳部组织,剪成小块后用70%乙醇进行消毒清洗,随后用含有0.25%胰酶和0.05% EDTA的消化液消化30 min,接着用0.2%胶原蛋白酶处理45 min.将分解的细胞放入含10% FBS的DMEM,置于离心机内1 500 r/min离心5 min,重复3次,最后将细胞放入装有培养液的60 mm培养皿中,置于38.5 ℃,5% CO2的培养箱中培养7~10 d,收集汇合后的单层细胞用消化液进行消化传代.一般用4~10代的成纤维细胞作为核移植供体,每次使用前将细胞消化成独立的单个细胞.
-
核移植操作参考之前报道的步骤[6]并进行了一些改变.在直径为60 mm培养皿盏中央做4个60 μL左右的操作滴,并在4个滴正上方做2个15 μL液滴,一个为显微操作液,另一个为胰酶消化液,再用矿物油覆盖.将成熟卵母细胞转入不含钙离子的显微操作液.在显微镜下利用盲吸法去核,用直径为25 μmol/L的操作针从3点钟方向进针并吸取1/5包含有第一极体和卵母细胞核的胞质.挑选合适的供体细胞,用注核针吸取注入受体卵周隙,点压透明带,使供体细胞与受体卵的胞膜接触紧密,随后将注核的卵母细胞转移到PZM-3中,在38.5 ℃,5% CO2,100%湿度培养箱中恢复1~1.5 h.随后将注入细胞核的卵母细胞用融合/激活液洗涤3遍并在其中平衡2 min,每10个放入铺满融合/激活液的融合槽(电极间距为500 μmol/L)内,用拉制的玻璃钝头针(直径100 μmol/L)拨动重组卵,使供体细胞-受体卵细胞膜接触面与电极平行.用83 V/mm,100 μs,1次直流脉冲的参数进行融合并且激活. 1 h后在显微镜下判定融合,重构胚用PZM-3清洗3遍置于PZM-3微滴后移入培养箱培养. 48 h统计卵裂率,168 h统计囊胚率.
-
分别收集不同时期猪卵母细胞和核移植胚胎,用4%多聚甲醛4 ℃固定过夜(8~12 h),固定后样品用阻断液阻断3次,每次5 min;室温条件下用1% Triton X-100透化30 min,随后用阻断液阻断2次,每次5 min;处理好的样品用1% BSA封闭1 h,随后样品分别移入H3K4me3和H3K4me9一抗中进行孵育,4 ℃过夜;样品取出后用清洗液清洗3次,每次5 min;随后放入装有相应二抗的离心管中避光作用1.5 h;最后用5 μg/mL碘化丙啶(PI)复染DNA 10 min,取出清洗液洗涤2次;样品放置于载玻片上,加入抗淬灭剂并用凡士林封片,使用相同拍照参数在激光共聚焦显微镜下拍摄荧光图片,使用Image Pro-plus 6.0软件计算荧光强度值.每组样品至少进行3次重复,每次至少检测5个囊胚,10个卵母细胞和其他阶段的胚胎,将GV期卵母细胞荧光强度值校正为1,随后进行相对荧光强度分析.
1.1. 主要试剂和仪器
1.2. 卵母细胞的收集及体外成熟
1.3. 供体细胞的准备
1.4. 核移植、融合以及激活
1.5. 单细胞免疫荧光分析
-
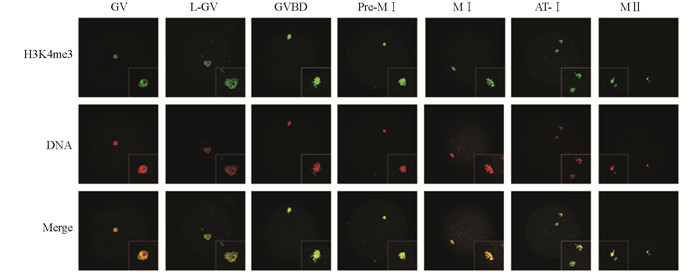
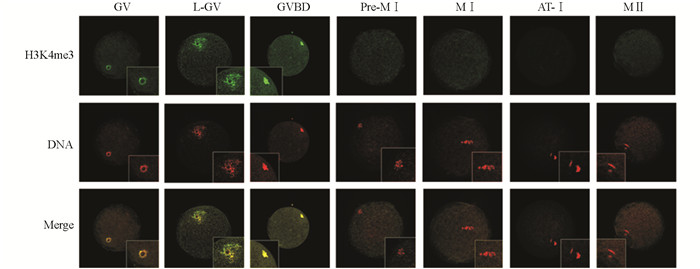
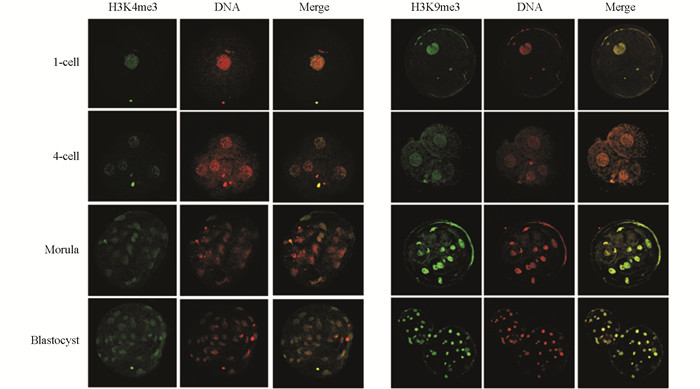
分别收集体外成熟不同发育阶段猪卵母细胞和克隆胚胎进行免疫荧光染色,得到H3K4me3和H3K9me3在猪卵母细胞体外成熟过程中的分布情况.结果显示,H3K4me3在GV至MⅡ阶段卵母细胞中均有表达,并且呈现出较强的荧光信号(图 1);H3K9me3只在GV,L-GV和MⅡ阶段卵母细胞中检测到明显的荧光信号,在其他阶段的卵母细胞中未检测到H3K9me3荧光信号(图 2);而在一细胞、四细胞、桑椹胚和囊胚期的体细胞克隆胚胎中均能检测到H3K4me3和H3K9me3荧光信号(图 3).
-
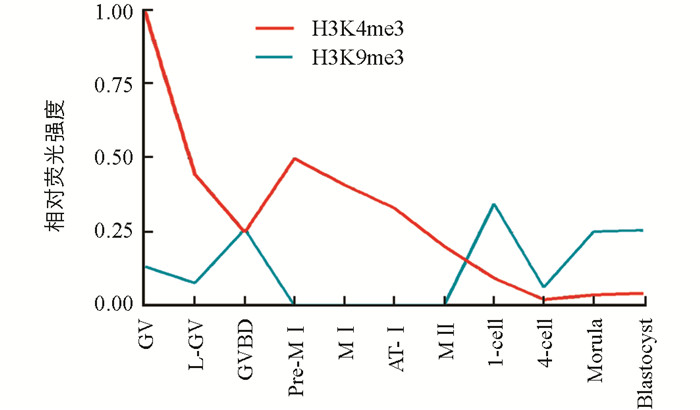
为了更好地分析H3K4me3和H3K9me3在猪卵母细胞和克隆胚胎中的表达模式,笔者对获得的细胞免疫荧光结果进行了相对荧光强度分析.结果显示(图 4),随着减数分裂和胚胎发育的进行,H3K4me3的表达呈现出逐渐下降的趋势,并且在四细胞胚胎中表达最弱. H3K9me3在GV至GVBD阶段卵母细胞中的表达呈现出上升的趋势,但是从Pre-MⅠ阶段开始荧光信号消失,直到一细胞克隆胚胎中荧光信号重新出现并且持续表达至囊胚阶段.此外,H3K9me3在卵母细胞中的相对荧光强度均低于H3K4me3;而在不同时期克隆胚胎中的相对荧光强度均高于H3K4me3.
2.1. 体外成熟猪卵母细胞和克隆胚胎中H3K4me3和H3K9me3的分布
2.2. H3K4me3和H3K9me3在猪卵母细胞和克隆胚胎中的表达模式
-
以往的研究者们已经对小鼠、猪、牛以及人类卵母细胞中组蛋白H3和H4中赖氨酸和精氨酸残基位点的甲基化进行了研究,结果发现这些甲基化位点都定位在哺乳动物卵母细胞染色体上.在小鼠、猪和牛的GV,Pre-MI和MII阶段卵母细胞中均能发现H3K4和H3K9二甲基化的表达[7-9],但鲜有研究H3K4和H3K9三甲基化在猪卵母细胞成熟过程的表达模式.本研究结果发现,猪卵母细胞不同成熟时期,H3K三甲基化均有表达,并且随着减数分裂进行,其呈现逐渐减弱的趋势,提示H3K4三甲基化调控的猪卵母细胞基因激活作用随着卵母细胞的成熟进程而不断减弱. H3K9二甲基化在Pre-MI和MII阶段猪卵母细胞中表达[8],本研究结果显示,H3K9三甲基化只在GV,L-GV和GVBD卵母细胞中表达,这与牛卵母细胞的表达模式相近[10],说明H3K9三甲基化可能并不直接参与猪卵母细胞Pre-MI及其随后成熟阶段的基因转录抑制调控.
组蛋白甲基化在供体核的重编程过程中起到重要的作用,高水平表达的组蛋白乙酰化有助于提高体细胞克隆胚胎的发育效率[11-12].近来的研究发现,体细胞克隆胚胎早期发育过程中存在组蛋白甲基化重编程异常,例如H3K9甲基化、H3K36三甲基化以及H3K4三甲基化等修饰在不同的哺乳动物克隆胚胎中均存在异常表达[13-15]. GAO等人研究发现,H3K4me3在体内发育的一细胞和四细胞猪胚胎中有较高表达,随着胚胎发育,其呈现出显著下降的趋势[16].猪早期胚胎一至四细胞阶段是母源向合子效应阶段转变的重要时期,此时合子基因组需要激活,而H3K4me3能够激活合子基因表达.本研究发现,不同发育时期猪克隆胚胎中,H3K4me3表达水平均较弱,推测一细胞和四细胞猪克隆胚胎中H3K4me3的异常表达可能影响合子基因组的激活,从而导致克隆胚胎发育效果降低.克隆牛胚胎中H3K9的过高甲基化通常伴随过高的DNA甲基化,且都高于正常胚胎中的表达水平[17],这提示H3K9甲基化在克隆胚胎中存在异常表达.在小鼠的克隆胚胎中也发现,早期分裂阶段中过多的H3K9me3积累不利于供体细胞核重编程,通过干扰H3K9me3的表达水平,可以提高克隆胚胎的发育效果[15].本研究结果显示,H3K9me3在一细胞,桑葚胚和囊胚阶段猪克隆胚胎中都存在较高的表达,推测至少在猪的克隆胚胎中,一细胞甚至更早阶段的胚胎发育阶段中存在过多H3K9me3积累,这可能是造成猪克隆胚胎发育效率低下的原因之一.该结果提示我们可以通过修正H3K4me3和H3K9me3表达水平来提高猪卵母细胞体外成熟效果和克隆效率. H3K4me3参与调控猪卵母细胞体外成熟发育整个过程,H3K4me3和H3K9me3与猪体细胞核移植胚胎体外发育有关,它们可能在体外成熟猪卵母细胞和体细胞克隆胚胎发育中表达异常.






 DownLoad:
DownLoad: