-
甲藻(Dinoflagellate)是水生态系统中浮游植物的重要组成部分,也是形成赤潮和水华的主要种类之一[1].甲藻形成的赤潮已经被广泛报道[2],但是对淡水甲藻水华的研究则相对较少.近年来,随着水体富营养化的加剧,已有大量淡水甲藻水华事件的报道[3-4].关于淡水甲藻水华的发生,存在着许多争议.有些研究表明水体富营养化直接导致了甲藻水华的发生[5],但有研究发现甲藻水华也会发生在中营养或贫营养水体中,与水体富营养化并没直接关系[6-7].因此,探讨营养盐对甲藻水华的发生则具有重要的意义.
营养盐浓度是影响浮游植物生长的重要因素,它不仅影响浮游植物生长速率、生化组成,而且影响浮游植物群落结构及空间格局[8-10].磷是植物的必需元素,它不仅参与植物生长发育过程中的各种代谢活动,而且也影响着植物细胞膜结构、信号传导和光合作用以及某些酶的活性调节等[11].无机磷因能被藻类直接吸收利用,被认为是藻类生长、繁殖最重要的磷源.然而,水体中的磷主要以颗粒态的形式存在,能被藻类直接利用的无机磷质量浓度往往少于水体中总磷质量浓度的5%,因此,磷已被认为是淡水水体中最主要的限制性元素[12-13].通过对野外甲藻水华的监测以及前人研究表明,磷的质量浓度与甲藻生物量之间呈显著正相关性[14].然而,磷如何影响甲藻的生长及水华的发生则较少研究.
因此,本研究以淡水甲藻-多甲藻为对象,通过叶绿素荧光动力学曲线(OJIP)及相关参数的变化,分析不同无机磷质量浓度对多甲藻光合作用过程中的能量流动、电子传递的影响,旨在探讨多甲藻生长及光合系统PSⅡ对不同磷质量浓度的响应,为进一步研究淡水甲藻水华发生及过程提供重要依据.
HTML
-
实验所用多甲藻(Peridinium umbonatum)来自中国科学院武汉水生所淡水藻种库.藻种置于119培养基(表 1)中进行培养,培养条件为温度(25±1) ℃,光强30 μmol/(m2·s),光照周期为12L:12D.到达对数期后,置于离心机中4 000 r/min离心5 min,弃上清液,用无磷的119培养基清洗,以去除表面吸附的磷.重复洗涤,离心3次后,转入无磷119培养基中,进行磷饥饿培养48 h后作为实验用藻.
配制5种不同质量浓度无机磷的119培养基(表 1),其中称取磷酸二氢钾磷(>99.5%)配置磷源母液,添加不同体积的磷源母液使培养基中最终磷质量浓度分别为0 mg/L,0.005 mg/L,0.02 mg/L,0.1 mg/L和0.6 mg/L.将磷饥饿培养后的甲藻等体积接入各个培养基中,在上述条件下培养,每个处理设置3个重复.
-
从接入藻种的初始天开始每2 d取藻液5 mL,用紫外分光光度计(UV-2550,岛津)在451 nm波长下测定其吸光度(OD).
-
每个处理各取2.5 mL对数期的藻液,经20 min的暗适应后,用植物效率分析仪(Handy PEA/LPA2,Hansatech)测定OJIP曲线,测定光强为3 000 μmol/(m2·s),测定结果包含了能够准确记录O-J-I-P等阶,即从10 μs到2 s的叶绿素荧光变化[15].通过OJIP曲线的变化及计算,获得了以下参数:F0,Fm,Fv,VJ,Sm,DI0/RC,TR0/RC,RE0/RC,ABS/CS0,DI0/CS0,TR0/CS0,ET0/CS0,RE0/CS0和PIABS.获得的OJIP曲线相关参数及其含义如表 2[16].
-
所有实验数据的处理和分析均在SPSS 17.0中进行,采用方差分析进行数据统计,p<0.05为差异有统计学意义.用Origin6.1绘制文中所有图形.
1.1. 藻体培养
1.2. 生长指标测定
1.3. 叶绿素荧光的测定
1.4. 数据处理及分析
-
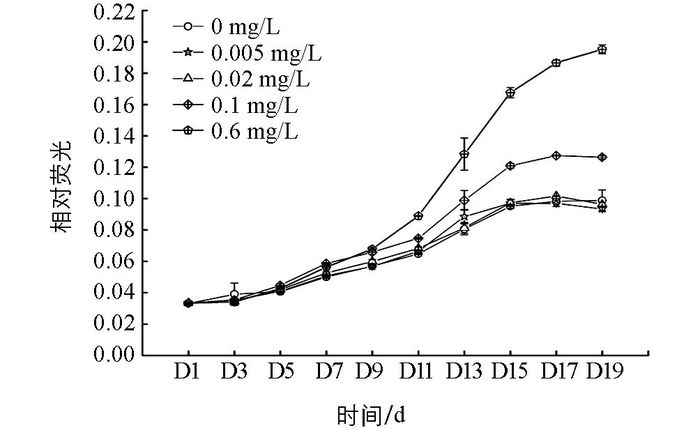
在不同质量浓度无机磷培养下,多甲藻的OD值随培养时间的增加而增加.整个培养期间,磷质量浓度为0.005 mg/L和0.02 mg/L的处理组与无磷条件相比差异无统计学意义.而培养的第11 d开始,0.1 mg/L和0.6 mg/L处理组的OD值高于无磷处理组(图 1).
-
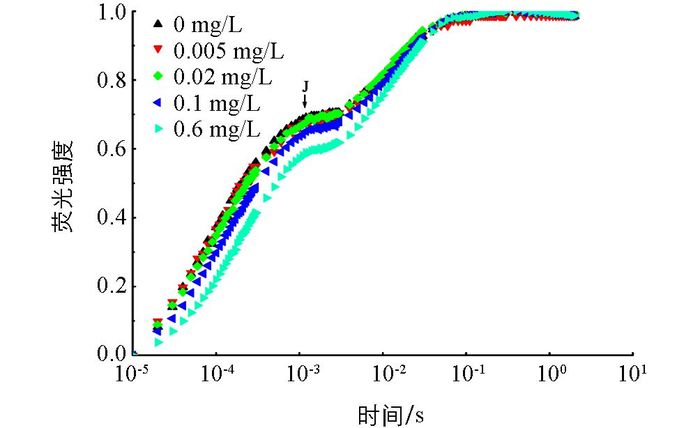
在不同质量浓度磷的培养下,多甲藻的OJIP曲线的变化如图 2.培养于0.6 mg/L磷下的多甲藻的相对可变荧光没有显著变化,与0.6 mg/L磷培养条件下的多甲藻荧光相比,培养于0,0.005 mg/L,0.02 mg/L和0.1 mg/L下的多甲藻OJIP曲线的J点均升高.虽然不同磷质量浓度下的OJIP曲线有差异,但其O,J,I和P四相均存在.
-
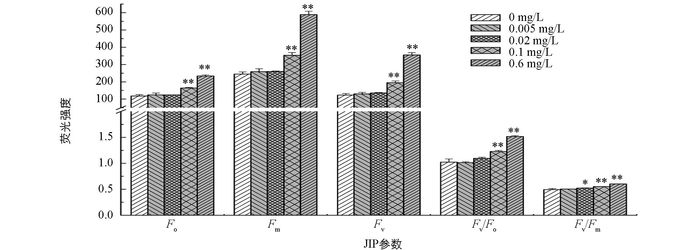
随着磷质量浓度的增加,F0,Fm,Fv,Fv/F0和Fv/Fm均增加,但与无磷条件相比,低磷环境(0.005 mg/L和0.02 mg/L)培养下的多甲藻的荧光参数差异无统计学意义,而富磷条件(0.1 mg/L和0.6 mg/L)下的荧光参数值则显著高于无磷条件(p<0.05)(图 3).
-
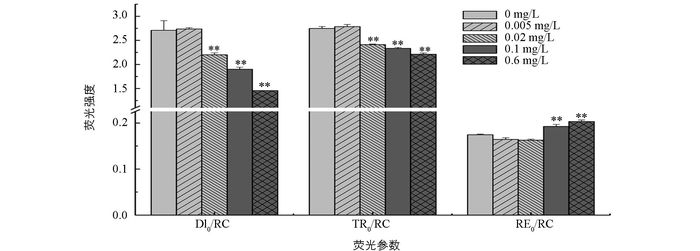
随着磷质量浓度的增加,DI0/RC逐渐降低,与无磷条件相比,磷质量浓度为0.02,0.1和0.6 mg/L时差异有统计学意义,DI0/RC分别降低了18.8%,29.8%和46.2%,磷质量浓度为0.005 mg/L时差异无统计学意义. TR0/RC的变化趋势与DI0/RC相似,随着磷质量浓度的增加而降低,且磷质量浓度为0.02,0.1和0.6 mg/L时差异有统计学意义,分别降低了12.2%,15.1%和19.5%. RE0/RC与DI0/RC和TR0/RC变化趋势相反,随着磷质量浓度的增加而增加,并且与无磷条件相比,富磷条件下差异有统计学意义,而低磷条件差异无统计学意义(图 4).
-
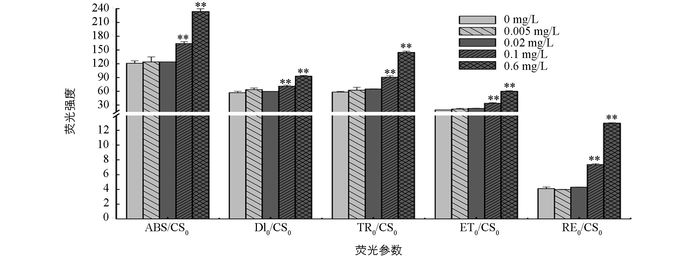
随着磷质量浓度的增加,ABS/CS0,DI0/CS0,TR0/CS0,ET0/CS0,RE0/CS0均增加.与无磷条件相比,低磷条件下各荧光参数差异无统计学意义(p>0.05),而富磷条件下的荧光参数差异有统计学意义(p<0.05).与无磷条件相比,两个富磷条件下的ABS/CS0分别增加了35.5%和93.4%;DI0/CS0分别增加了24.9%和63.4%;TR0/CS0分别增加了56%和149.3%;ET0/CS0分别增加了73.4%和208.3%;RE0/CS0分别增加了80%和216%(图 5).
-
不同磷质量浓度下VJ,Sm,PIABS,R0的变化如表 3.随着磷质量浓度的增加,VJ逐渐降低.与无磷条件相比,磷质量浓度为0.005 mg/L时,VJ无显著变化,而当磷质量浓度为0.02 mg/L或大于0.02 mg/L时,VJ显著降低. Sm,PIABS和R0均随着磷质量浓度的增加而增加.与无磷质量浓度相比,富磷条件下的荧光参数显著增高,而低磷条件下差异无统计学意义.
2.1. 不同磷质量浓度对多甲藻生长的影响
2.2. 磷对叶绿素荧光诱导动力学曲线(O-J-I-P)的影响
2.3. 叶绿素荧光参数分析(JIP-test)
2.3.1. 磷质量浓度对多甲藻F0,Fm,Fv,Fv/F0和Fv/Fm的影响
2.3.2. 不同磷质量浓度对单位反应中心能量流动的影响
2.3.3. 不同磷质量浓度对单位反应面积能量流动的影响
2.3.4. 不同磷质量浓度对VJ,Sm,PIABS和R0的影响
-
有研究表明磷能影响微藻的生长速率和光合作用[17-18],当磷质量浓度过低时,微藻的生长会受到严重抑制[19],我们的研究结果也支持了这一结论(图 1).说明多甲藻的生长与磷质量浓度有直接的关系.
通过O-J-I-P荧光动力学分析可以得出各种指示光合系统Ⅱ能量分布、吸收、诱捕、耗散等参数及电子传递状况[16].在正常生理条件下,典型的快速叶绿素荧光诱导动力学曲线记录了从F0到Fm的整个荧光变化过程,即O,J,I和P四相[20-21].在本研究中,与超富磷处理组(0.6 mg/L)相比,其余磷处理组的J点均上升,说明低磷条件下,多甲藻的电子传递受到一定程度的抑制.
F0代表初始荧光,是不参与光化学反应的光能辐射部分,其与叶绿素浓度有关[22-23]. Fm和Fv分别表示最大荧光(光合系统Ⅱ反应中心原初电子受体全部还原时的荧光)和可变荧光(参与光化学反应的光能辐射部分)[22].在荧光的测定中,Fv/F0可反映PSⅡ活性,而Fv/Fm可代表PSⅡ光化学的最大效率[24].植物体内磷的质量浓度会影响其光能转换和利用[25-26].研究表明[17],当藻细胞遭受磷限制时,Fv/Fm会明显降低,其可能原因是低磷条件限制了藻细胞的光合磷酸化,降低了卡尔文循环效率,进而导致光合速率降低[27].本研究发现随着磷质量浓度的增加,F0,Fm,Fv,Fv/F0和Fv/Fm逐渐增加(图 3),表明磷质量浓度增加,多甲藻受激发的电子密度增加,用于进行有效光化学反应的光合辐射增加,进而导致多甲藻的光合效率增强,多甲藻生长速率增快.而无磷和低磷处理下多甲藻电子传递受阻,受到光合抑制.
植物在进行光合作用时,吸收的光能大部分用于光化学反应和光合能量的转换,只有少部分以热和叶绿素荧光等方式散失掉.一旦植物受到胁迫,其光化学反应降低,吸收的光能则更多以热和荧光形式损失掉[28-29].本研究发现,与高磷处理组相比,无磷与低磷处理组的荧光参数RE0/RC,ABS/CS0,DI0/CS0,TR0/CS0,ET0/CS0,RE0/CS0和R0均显著降低,而DI0/RC和TR0/RC显著升高(图 4,图 5).说明在无磷和低磷处理下,多甲藻光合系统Ⅱ中有活性反应中心的数量及单位面积吸收的光能减少,而用于还原QA的能量增加,使得单位面积电子传递产额降低,传递到电子链末端的能量也降低,进而导致整个光合电子传递影响.此外,无磷和低磷处理组中DI0/RC的升高,进一步说明单位反应中心耗散掉的能量增加,而用于光化学反应的能量相应减少,光能有效利用率降低,导致光合作用受抑制.相反,在高磷处理下,多甲藻热耗散降低,并且用于电子传递的能量增加,使得光能利用率增加,光合效率增大.
VJ是指光照2 ms时有活性的反应中心关闭程度[16]. Sm则表示QA全部被还原时所需要的能量,即PSⅡ受体侧PQ库的大小,电子从QA-进入电子传递链越多,Sm的值就越大[16].由表 3可以看出,在无磷和低磷处理条件下,VJ和Sm显著低于高磷处理组,说明磷限制使得RC的数量减少,获得的能量较少用于电子传递而更多用于还原QA,从而抑制了QA到QB的电子传递,造成QA-大量积累,降低了PQ库容量[30],J点升高[31]. PIABS是反映基于光能吸收的性能指数,因此能很好地表征植物光合结构的状态[32-33]. PIABS随着磷质量浓度的降低而降低(表 3),表明磷的降低,影响了多甲藻的光合结构.
综上所述,在不同无机磷质量浓度处理下,高磷处理显著提升了多甲藻的光合效率,而无磷和低磷处理则显著抑制了光合电子传递,降低了光合效率.因此,随着水体磷质量浓度的升高,多甲藻的光合效率增加,将促使其生物量的增加,为其水华的发生提供前提条件.






 DownLoad:
DownLoad: