-
低温是一种重要的非生物胁迫因素,严重影响不耐寒作物的产量和品质[1].拟南芥等耐寒植物在遇到零上低温时具有冷适应(cold acclimation)机制,使植株得到冷锻炼而具备耐受零下低温的能力[2],其中包括依赖ABA的冷信号转导途径和不依赖ABA的CBF冷应答系统[3].拟南芥CBF家族中有AtCBF1、AtCBF2、AtCBF3、AtCBF4、AtCBF5和AtCBF6等6个成员,但仅AtCBF1、AtCBF2和AtCBF3受低温诱导表达[4].这3个基因在拟南芥基因组中按AtCBF1-AtCBF3-AtCBF2的结构排列,是CBF冷应答系统的关键节点,调控着下游众多的冷调节(Cold-regulated,COR)基因的表达,构成了一个庞大的CBF调节组,在拟南芥的耐寒能力获得中起着十分重要和不可缺失的作用[4-5];其中,AtCBF2除了能激活下游COR基因表达外,还具有抑制AtCBF1和AtCBF3表达的特性,以便拟南芥能以最低水平的转录调控来获得最大程度的耐寒能力[6-7]. AtCBF1或AtCBF3在拟南芥中超表达[8],将它们单独导入烟草、马铃薯等其他物种中[9-10],均能显著提高转基因植物的耐寒性. CBF冷应答系统已被证实在植物界普遍存在[4].在冷敏植物番茄中,同样存在CBF冷应答系统[11],并以SlCBF1、SlCBF2和AtCBF3基因簇的结构存在,但只有SlCBF1能够应答低温,表明番茄中只存在不完善的CBF冷应答系统[11].本研究将拟南芥AtCBF1-AtCBF3-AtCBF2基因簇整体转入番茄基因组,分析它们在番茄中的低温诱导表达特性和对番茄耐寒性的影响,以期修正番茄的CBF冷应答系统的遗传缺陷,提高番茄的冷应答能力和耐寒性,为培育优异的番茄耐寒育种材料奠定基础.
HTML
-
番茄(Solanum lycopersicum L.)品种Ailsa Craig、大肠杆菌菌株XL1-blue和根癌农杆菌(Agrobacterium tumefaciens)菌株LBA4404均由西南大学蔬菜实验室提供.含有拟南芥AtCBF1、AtCBF2和AtCBF3基因(均带自身的启动子;而AtCBF1和AtCBF3带自身的终止子,AtCBF2未带)的双元载体pBK-AtCBF1~3(图 1A)由该实验室构建. pMD18-T载体购自TaKaRa公司.引物(表 1)委托上海生工生物技术有限公司合成.
-
选取籽粒饱满的番茄种子,在净化工作台上用75%酒精浸泡60 s,再用20%新配制的NaClO溶液表面消毒20 min,无菌水漂洗5~6次后,均匀地播种于MS[12]培养基上,置于(25±2) ℃、16 h光照/8 h黑暗的培养室内培养约7 d.当番茄子叶刚刚展开时,在滤纸上用灭过菌的解剖刀切取子叶,置于含有1 mg/L吲哚乙酸(IAA)、1.75 mg/L玉米素(ZT)、3%蔗糖和0.6%琼脂的MS固体预培养基上,(25±2) ℃暗培养2 d.
双元载体pBK-CBF1~3经冻融法[13]转化根癌农杆菌菌株LBA4404.将活化后重悬的农杆菌菌液倒入培养皿中,侵染番茄子叶外植体10 min左右,外植体经无菌滤纸吸去多余的菌液后,重新放回到预培养基上,(25±2) ℃暗培养2 d后,转移到含有1 mg/L IAA、1.75 mg/L ZT、75 mg/L卡那霉素(Kan)、500 mg/L羧苄青霉素(Cb)、3%蔗糖和0.6%琼脂的MS筛选培养基上,(25±2) ℃每天16 h光照条件下培养,每15 d换一次筛选培养基直至获得Kan抗性试管苗.切下的试管苗在50 mg/L Kan、200 mg/L Cb、3%蔗糖和0.6%琼脂的MS生根培养基诱导生根.生根的Kan抗性植株转移到无菌土中炼苗,并在(25±2) ℃、每天16 h光照的育苗室内培养.
-
用CTAB法提取转基因番茄和野生型番茄的gDNA[14].用P1和P2、P3和P4、P5和P6、P7和P8等引物对(表 1、图 1A)进行PCR扩增,检测具有卡那霉素抗性的新霉素磷酸转移酶基因nptII和AtCBF1~3基因是否整合进番茄基因组.将PCR阳性的转基因番茄移植到网室内培养留种,播种后长出的T1代植株再经PCR扩增鉴定后,选取形态正常的转基因株系,自交得到T2代,经PCR鉴定出纯合转基因株系,用于后续的耐寒性和转基因表达水平的检测.
-
转基因番茄(T2代)和野生型番茄植株,在25 ℃、16 h光照和22 ℃、8 h黑暗的光照培养箱内培养,待其长到五叶一心时,选取长势一致的番茄植株在低温光照培养箱中4 ℃处理0~3 d,每个时间点每个株系各3个植株,观察转基因的耐寒性.采用电导仪方法测定叶片组织的相对电导率[15].采用硫代巴比妥酸法测定丙二醛(MDA)的含量[16].采用钼酸铵比色法测定过氧化氢酶(CAT)活性.采用愈创木酚法测定过氧化物酶(POD)活性.采用邻苯三酚自氧化法测定超氧化物歧化酶(SOD)活性.采用考马斯亮蓝法测定总蛋白含量[17].
-
转基因番茄(T2代)和野生型番茄植株,在上述光照培养箱内培养至五叶一心的幼苗时,选取长势一致的植株在低温光照培养箱内4 ℃处理0~6 h,取第4片真叶,提取总RNA,并反转录成cDNA.根据AtCBF1~3 cDNA序列设计RT-PCR引物(表 1),以番茄的elongation factor 1-alpha (LeELF-α,GenBank登陆号为X14449) 基因作为内参基因(表 1),采用Bio-Rad CFX96荧光定量PCR仪进行扩增. 10 μL PCR体系中含有5 μL的SYBR Premix Ex Taq、3 μL H2O、1 μL cDNA、上下游引物各0.5 μL;每个样重复3次. PCR扩增程序为:95.0 ℃ 3 min;95.0 ℃ 30 s;60.0 ℃ 30 s;72.0 ℃ 30 s;40个循环;95.0 ℃ 5 s.根据相对定量公式2-ΔΔCt(ΔCt=Ct AtCBF1-CtActin,ΔCt=Ct AtCBF2-CtActin,ΔCt=Ct AtCBF3-CtActin)计算,分析不同样品的目的基因相对表达量.所有的数据采用Spass19.0软件进行数据统计分析,并用Excel作图.
1.1. 材料
1.2. 方法
1.2.1. 农杆菌介导法转化番茄
1.2.2. 转基因番茄的分子鉴定
1.2.3. 转基因番茄的耐寒性鉴定和理化指标分析
1.2.4. AtCBF1~3转基因表达的定量RT-PCR分析
-
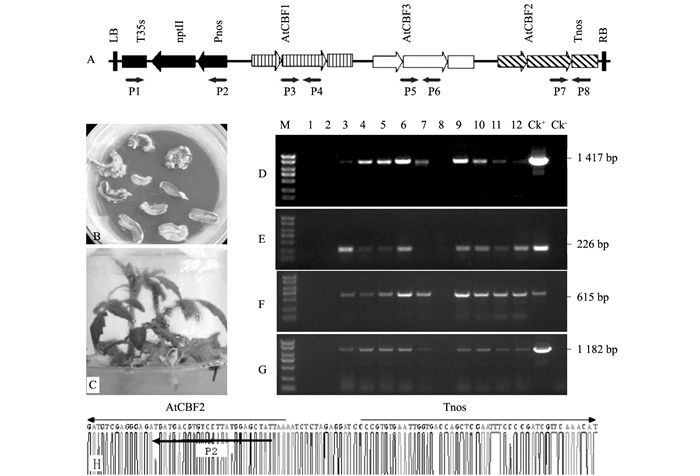
双元载体pBK-CBF1~3经冻融法转化根癌农杆菌菌株LBA4404,转化番茄子叶外植体后,4~6周左右得到Kan抗性不定芽(图 1B);在不加激素的培养基上诱导生根,形成试管苗(图 1C).共得到18株卡那霉素抗性植株.提取gDNA,经P3和P4、P5和P6、P7和P8引物对进行PCR扩增检测,其中8株转基因番茄中分别可扩增到目的基因AtCBF1、AtCBF3和AtCBF2基因的片段,扩增产物的大小约为1 417 bp、615 bp、226 bp(图 1D、E、F);同时,经P1和P2引物对也能够扩增出1 182 bp大小的Kan抗性基因Pnos-nptII-T35s(图 1G);均与预期阳性对照的大小一致,而阴性对照没有扩增出目的条带(图 1D、E、F、G). P7和P8引物对的PCR扩增产物经测序鉴定,含有预期的AtCBF2基因和Tnos终止子(图 1H).因此,证实外源基因已经整合到番茄基因组.在自交T2代群体中,经PCR扩增筛选到了N1和N2两个转基因纯合株系.
-
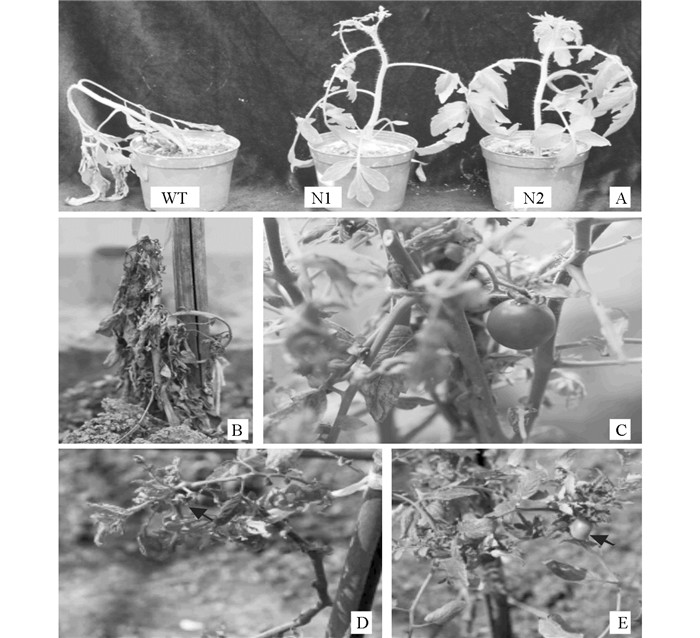
25 ℃生长的转基因番茄和野生型材料经4 ℃低温处理3 d,可明显看到野生型植株萎焉,而转基因番茄虽然叶片下垂,但植株挺立,表现出比野生型植株有更强的耐寒性(图 2A).秋季播种的野生型番茄在1月初(室外最低温度为2 ℃左右)即受冻死亡(图 2B),而同期播种的转基因番茄虽然下部叶片受冻后枯死,但顶叶及紧随其下的一些叶片保持翠绿,果实能够红熟(图 2C),而且能够越过最寒冷的2月(1 ℃左右),并在3月中旬重新开花(图 2D)和3月下旬结实(图 2E),比常规冬季育苗后春季露地普通番茄栽种的开花结实提早1月左右.因此,整合有拟南芥AtCBF1、AtCBF2和AtCBF3的转基因番茄的耐寒性得到明显增强.但该转基因番茄置于0 ℃低温条件下1 d即发生萎焉和死亡,表明拟南芥AtCBF1-AtCBF3-AtCBF2基因簇并不能赋予转基因番茄耐冻性
-
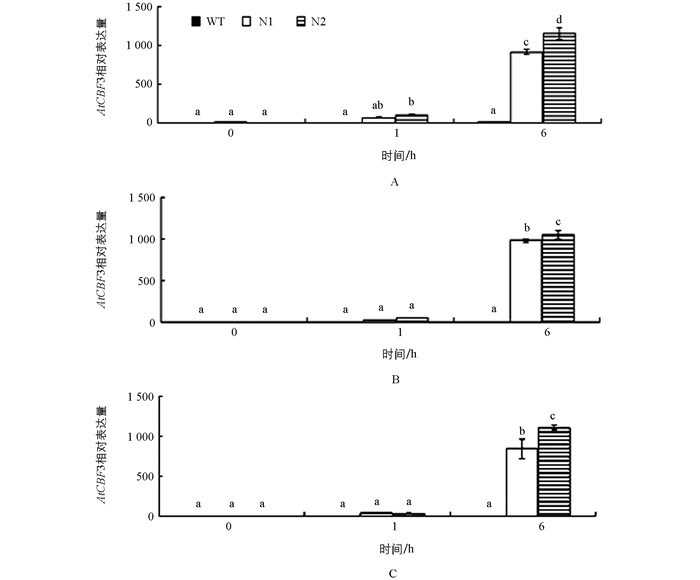
转基因番茄在室温生长条件下几乎没有检测到AtCBF1~3基因的表达,而这3个基因经4 ℃处理1 h时表达量增高,6 h达到丰量水平,其中AtCBF1在N1和N2株系分别增加了83和145倍,AtCBF2分别增加了200和211倍,AtCBF3分别增加了410和575倍,且与野生型材料和转基因材料之间在未经低温处理和4 ℃1 h时均存在显著性差异(图 3),表明拟南芥AtCBF1、AtCBF2和AtCBF3基因在番茄中也能感应低温,受低温诱导表达;而且3个基因的冷诱导表达模式相似(图 3).因此,可以推测,转基因番茄的耐寒性增强与外源基因AtCBF1、AtCBF2和AtCBF3基因受低温诱导后丰量表达有关.
-
表 2表明,4 ℃低温胁迫使野生型番茄和转基因番茄株系N1、N2的相对电导率(REC)有不同程度的增加.在25 ℃条件下,野生型和转基因番茄之间的相对电导率无明显差异,但在冷胁迫处理1 d和3 d时,野生型番茄的相对电导率均显著高于转基因番茄株系N1和N2.这些结果表明,与野生型番茄相比,转基因番茄的细胞膜损坏程度较轻,表明AtCBF1~3的异源表达提高了转基因番茄细胞膜的稳定性.
在25 ℃生长条件下,MDA含量在野生型番茄和转基因番茄之间差异不明显(表 3).随着低温胁迫时间的延长,转基因番茄株系N1和N2的MDA含量虽然略有增加,但与0 h相比,均未出现显著性变化;而野生型番茄在低温处理3 d时,与0 h或与转基因番茄株系之间的MDA含量差异达到了显著水平(表 3).结果表明,低温胁迫处理后,野生型番茄的膜脂质过氧化程度更为严重,而转基因番茄对低温的适应能力得到增强.
-
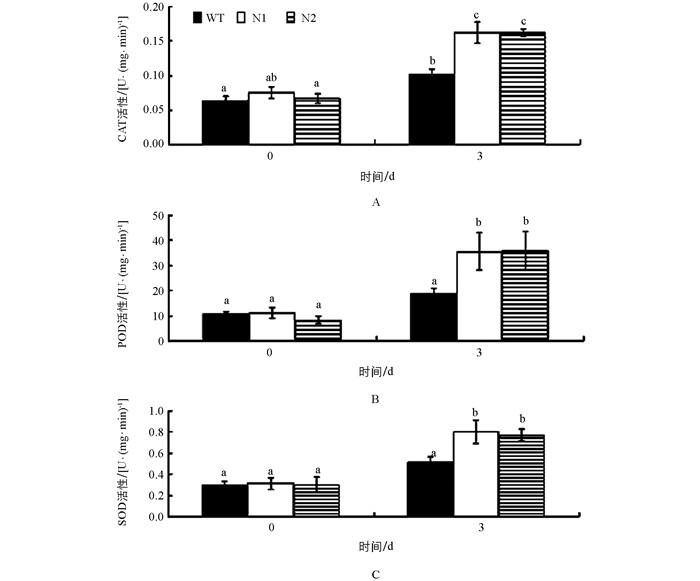
当植物受到低温逆境胁迫时,产生的活性氧作为第二信使诱导了植物体内抗氧化酶(CAT、POD、SOD等)的活性升高.抗氧化酶能清除活性氧自由基并使之保持较低水平,维持细胞膜的稳定性和完整性,使得植物对低温具有更强的耐受性. 图 4表明,在25 ℃正常生长条件下,转基因番茄(N1、N2) 与野生番茄(WT)的过氧化氢酶(CAT)、过氧化物酶(POD)和超氧化物歧化酶(SOD)的活性无明显差异,但在4 ℃低温胁迫3 d后,转基因番茄的CAT、POD和SOD的活性显著高于野生型番茄.结果表明,与野生型番茄相比,低温胁迫下转基因番茄由于AtCBF1~3的异源表达而使抗氧化酶活性显著性增高,增强了转基因番茄的耐寒性.
2.1. AtCBF1~3基因整合进了番茄基因组
2.2. AtCBF1~3基因增强了番茄的耐寒性
2.3. AtCBF1~3基因在转基因番茄中能受低温诱导表达
2.4. AtCBF1~3基因表达增强了低温胁迫下番茄细胞膜的稳定性
2.5. AtCBF1~3基因表达增强了低温胁迫下番茄抗氧化酶的活性
-
番茄属于冷敏植株,缺乏拟南芥那样完善的冷适应机制,其CBF冷应答系统为部分保守,仅SlCBF1单个转录激活因子在该系统中发挥冷调控作用[11].本研究成功地将拟南芥AtCBF1-AtCBF3-AtCBF2基因簇导入番茄基因组(图 1A、D、E、F),而且3个基因均能在转基因番茄中受低温诱导后6 h高丰量表达(图 3),为深入研究AtCBF基因在番茄中的调控模式和调控的基因网络奠定了基础.在拟南芥中,AtCBF1和AtCBF3分别受控于上游ICE2和ICE1转录因子的调控[18],但番茄中仅鉴别到1个SlICE1基因[19];因此,SlICE1如何能同时调控3个AtCBF的表达,以及AtCBF2如何参与调控AtCBF1和AtCBF3,仍有待研究.
导入拟南芥AtCBF1-AtCBF3-AtCBF2基因簇的转基因番茄的叶片的电解质渗透率和膜脂过氧化程度显著低于野生型对照(表 2、表 3),CAT、POD、SOD的活性也显著高于野生型材料(图 4),表明AtCBF1、AtCBF2和AtCBF3基因能够通过提高转基因番茄的活性氧清除能力,保护细胞膜系统,从而使转基因番茄比野生型材料具有更强的耐寒性(图 2).此外,含有AtCBF1-AtCBF3-AtCBF2基因簇的转基因番茄并不能耐受0 ℃左右的低温,这是由于番茄的起源和进化过程中没有像耐冻的拟南芥那样构建出庞大的COR基因家族[11].这些结果与单独导入CaMV 35S启动子控制的AtCBF1基因导入番茄后的结果相一致[20].因此,番茄的耐寒性改良尚需要导入大量能够增强耐寒性的COR基因来实现.






 DownLoad:
DownLoad: