-
谷胱甘肽(Glutathione,GSH),即γ-L-谷氨酰-L-半胱氨酰-甘氨酸,是一种由L-谷氨酸、L-半胱氨酸和甘氨酸经肽键缩合而成的生物活性三肽,也是一种广泛存在于生物体内的非蛋白硫醇化合物[1].自然界中谷胱甘肽以还原型(GSH)和氧化型(GSSG)2种状态存在,通常所说的谷胱甘肽是指还原型谷胱甘肽. GSH的主要特点是具有游离的巯基和很强的供电子或质子的能力,它可以作为水相的抗氧化剂和抗氧化酶的辅因子.这些结构特点决定了在生物体内发挥重要的生理功能:细胞内氧化还原活性的维护[2]、细胞内信号转换[3]、细胞的代谢[4]、基因调控[5].同时,GSH也广泛应用于医药、食品和化妆品行业.
目前,关于GSH的检测方法较多,如高效液相色谱法[6]、荧光法[7]、毛细管电泳法[8]和电化学检测法[9]等.对于GSH的检测,虽然报道的光学生物传感方法大部分比较简单,有较高灵敏度,但存在实验步骤繁琐、样品处理耗时等缺点.近年来,随着纳米技术的不断发展,纳米微粒作为模拟酶分析应用的研究引起了广泛关注.与天然酶相比,纳米酶具有一些优点:低成本的可控合成、高的催化活性、能够耐受更苛刻的环境等[10].但是,这些纳米材料的制备程序和修饰步骤复杂耗时,且在合成过程中容易团聚,合成的纳米材料也存在批间差异,从而使结果重现性低,本研究发现一种生物催化剂——木瓜蛋白酶具有类似过氧化物酶的性质.基于此,开发了一种新型的GSH检测方法来满足临床、生物和医疗需求.
木瓜蛋白酶(EC3.4.22.2) 是木瓜中含有的一种低特异性蛋白水解酶,属巯基蛋白酶,由212个氨基酸残基组成的一条肽链,活性位点为25位的半胱氨酸残基和158位的组氨酸残基[11].它具有耐高温、稳定性好和蛋白水解能力强等特征,在食品和医药等领域有广泛的应用.最近笔者所在研究组发现木瓜蛋白酶具有类似过氧化物酶的性质,能催化H2O2氧化过氧化物酶底物并产生特定的颜色反应[12].而本研究发现,GSH可以抑制木瓜蛋白酶催化TMB-H2O2显色反应.基于此现象,本研究建立了快速简单的方法用来高灵敏和高选择地检测GSH,该方法操作简单,不需要复杂的仪器设备,并成功用于实际样品中GSH的检测.
HTML
-
H2O2(30%)、磷酸氢二钠、磷酸二氢钠、葡萄糖、抗坏血酸、氢氧化钠、氯化钠、氯化钾、硫酸镁、硝酸钙、硫酸锌、硫酸铝、硫酸亚铁和硫酸铜购自重庆川东化工有限公司;3,3',5,5'-四甲基联苯胺(TMB)、丙氨酸(Ala)、异亮氨酸(Ile)、酪氨酸(Tyr)、甲硫氨酸(Met)、天门冬氨酸(Asp)、精氨酸(Arg)、缬氨酸(Val)、赖氨酸(Lys)、组氨酸(His)、亮氨酸(Leu)、苯丙氨酸(Phe)、谷氨酸(Glu)、甘氨酸(Gly)、胱氨酸(Cystime)和GSH购自上海生工生物工程有限公司;超滤管(截留分子量为5 kDa)购自Sigma-Aldrich公司;尿样为西南大学校医院提供;所有试剂均为分析纯,可直接使用;本实验用水均为Milli-Q-Plus超纯水系统所制备的超纯水(Millipore,18.2 MΩ,美国).
UV-2450紫外可见分光光度计(日本岛津公司);漩涡混合器(金坛市医疗仪器厂);高速台式冷冻离心机LRH-250-Z383K(德国HERMLE公司);电热恒温水浴槽(上海比朗仪器有限公司).
-
首先,配置10 mmol/L的GSH储备液,并通过梯度稀释获得不同浓度的GSH.再将200 μL 1.0 μg/mL木瓜蛋白酶与不同浓度的GSH于35 ℃孵育30 min,然后向混合液中分别加入6.0 mmol/L TMB,10 mmol/L H2O2和0.1 mol/L磷酸盐缓冲溶液(pH=5.0)200 μL,并加水至终体积为2 mL;混合均匀后,35 ℃继续孵育2 h,用紫外可见分光光度计测其在652 nm的吸光度.对于实际样品的检测,先将样品以超滤管3 000 r/min离心30 min,滤液以超纯水稀释一定倍数,代替上述的谷胱甘肽标准液,按同样的方法进行测定.
1.1. 试剂与仪器
1.2. GSH的检测
-
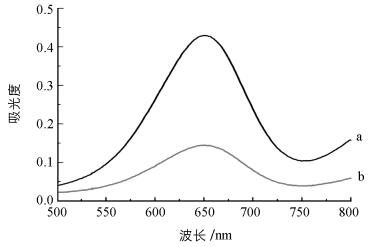
研究发现木瓜蛋白酶具有过氧化物酶活性,能催化H2O2氧化过氧化物酶底物TMB,产生蓝色反应,并在652 nm处有吸收峰(图 1);而GSH会消耗H2O2,导致吸光度值降低且溶液颜色变浅.基于此,本研究设计了一种比色分析法检测GSH,其检测原理为:
-
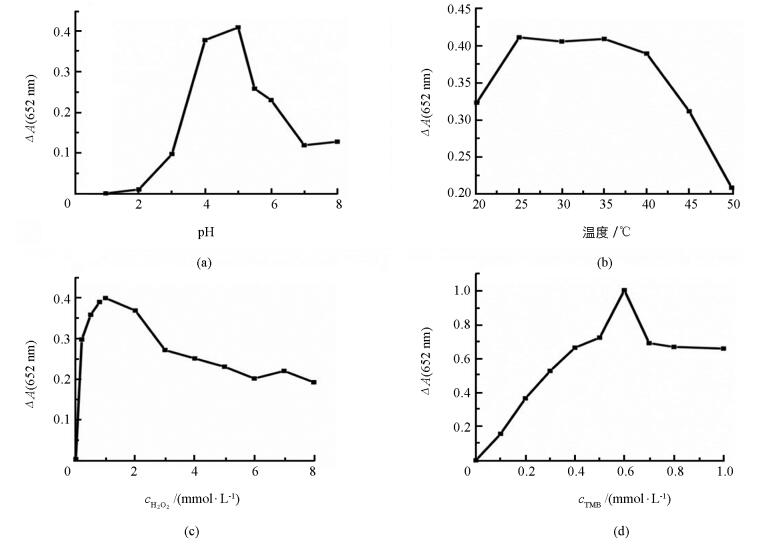
实验中优化了pH值、温度、H2O2浓度及TMB浓度对检测GSH的影响(图 2). 图 2中纵坐标ΔA=A0-A,其中A0和A分别表示在不加GSH和加入GSH的吸光度值.在1.0~8.0的范围内优化了pH值(图 2(a)):pH值从1.0逐渐增大时,ΔA也随之增大;当pH进一步增大时,ΔA逐渐降低.因此,5.0为最佳pH值.在20~50 ℃范围内优化了最佳温度:孵育温度在25~35 ℃之间,ΔA几乎没有差别;然而当温度超过35 ℃时,随着温度的升高,ΔA值降低(图 2(b)).因此,选择35 ℃为最佳反应温度.当H2O2浓度在0.10~1.0 mmol/L范围内时,ΔA值随着H2O2浓度的增大而增大;在1.0~8.0 mmol/L范围内,ΔA随着浓度的增大而降低,这是因为较高浓度的H2O2会抑制木瓜蛋白酶的催化活性(图 3(c)).因此,选择1.0 mmol/LH2O2浓度为最佳测定条件.在0.10~1.0 mmol/L范围内优化了TMB浓度(图 2(d)),当TMB浓度为0.60 mmol/L时,ΔA值达到最大,因此选择TMB浓度为0.60 mmol/L.
-
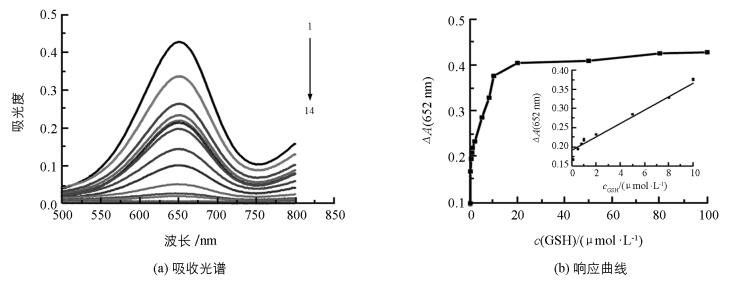
在最佳的实验条件下,将不同浓度的GSH加入反应体系中,通过检测木瓜蛋白酶-TMB-H2O2反应体系在652 nm的吸光度,实现对GSH的检测.对应光谱图和标准曲线(图 3),在0.10~10 μmol/L范围内,GSH浓度与吸光度具有良好的线性关系,其线性方程为ΔA=0.017 6c+0.190 9,相关系数为0.991 2,检出限为0.03 μmol/L.与其他方法比较(表 1),本方法具有较高的灵敏度.此外,本实验不需要合成纳米材料,也不需要复杂的仪器设备,且操作过程简单,表明该方法在生物技术、生物分析及生物医学上有潜在应用.
-
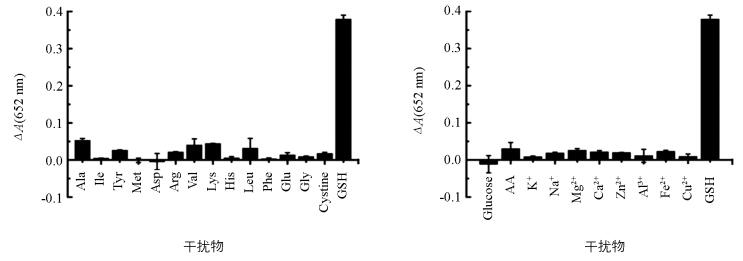
为了考察该方法的选择性,向木瓜蛋白酶-TMB-H2O2显色体系中分别加入5 μmol/L的葡萄糖、抗坏血酸、14种氨基酸(丙氨酸、异亮氨酸、酪氨酸、甲硫氨酸、天门冬氨酸、精氨酸、缬氨酸、赖氨酸、组氨酸、亮氨酸、苯丙氨酸、谷氨酸、甘氨酸、胱氨酸)、8种金属离子(K+,Na+,Mg2+,Ca2+,Zn2+,Al3+,Fe2+,Cu2+)等常见的干扰物,并在上述最佳条件下,测其在652 nm的吸光度(图 4).结果显示,同浓度的干扰物质均对显色体系无明显影响,表明本方法对GSH有很高的选择性.
-
为了证实该方法在实际应用中的可行性,检测了人尿液样品中GSH的含量.将尿液样品超滤处理后,并稀释至一定倍数,采用标准加入法进行检测,所得回收率为97%~109.4%,结果列于表 2中.表明该方法能够成功用于实际样品中GSH的分析检测.
2.1. 实验原理
2.2. 实验条件优化
2.3. GSH的检测
2.4. 方法选择性
2.5. 生物样品分析
-
基于木瓜蛋白酶的过氧化物酶活性设计了一种简便、灵敏地检测GSH浓度的比色分析法.木瓜蛋白酶可以催化H2O2氧化TMB产生蓝色反应,并在652 nm处有吸收峰;而当加入GSH后,由于GSH会消耗H2O2,导致在652 nm处的吸收峰强度降低. GSH检测的线性范围为0.10~10 μmol/L,检出限为0.03 μmol/L.该方法具有灵敏、简单、成本低廉且选择性良好的优点,可用于实际样品的检测.






 DownLoad:
DownLoad: