-
氮是植物生长发育所需重要元素之一,空气中约占80%的分子态氮,无法被绝大多数植物所利用,只能从土壤中吸收结合态氮.然而,土壤中的含氮化合物并非土壤本身固有,而是来自生物固氮的产物,包括土壤微生物固氮和豆科植物根瘤菌固氮.据估计,地球表面上,生物固氮总量约为70t/年;其中,豆科植物根瘤菌固氮量约占60%[1-5].
根瘤是豆科植物固氮的场所,是由固氮细菌在植物根系上建成的共生组织.根瘤菌不仅从根瘤细胞中摄取自身生活所需的养分,而且能固定游离氮、合成含氮化合物,供豆科植物生长发育所需的氮素.另外,由于根瘤的脱落和结有根瘤的根系遗留在土壤中,增加土壤的肥力.据研究,豆科植物中,类菌体分化和固氮体系形成与一些宿主基因有关[6-7];根瘤菌侵入根细胞后,宿主细胞的类黄酮诱导根瘤菌的nod基因表达、分泌出根瘤菌结瘤因子(Nod factor),进而引起宿主植物的根瘤形成[8].在豌豆中,sym31和sym32基因在根瘤的形成中具有被感染细胞识别入侵的根瘤菌的功能[9-10].虽然豌豆的Sym13基因被推定为在根瘤菌分化成类菌体时能够表达氮化酶蛋白的功能,但在根瘤中并未检测到氮化酶的活性[11].
百脉根是Stougaard等提倡的豆科植物分子遗传学研究的模式植物[12];国内外研究者采用多种诱变法获得了大量百脉根突变体.这些无效根瘤突变体的根瘤,不仅固氮能力下降明显,而且根瘤的早期老化现象严重[12-17];其中,结白色或绿色根瘤的无效根瘤突变体,被用于共生固氮体系的建立和宿主基因的研究中.根瘤菌侵入根细胞后建立共生关系的过程极为复杂,根瘤菌的Nod factor收容和早期信号传达后,根瘤菌感染和入侵细胞的过程、在被感染寄主细胞内建立共生关系、类菌体分化、共生根瘤的成熟及维持机制等诸多问题还有待进一步深入阐明.
本研究利用EMS诱变处理百脉根MG-20种子而获得的突变体库中筛选出的3种无效根瘤突变体[18]为材料,在前期研究的基础上,对所筛选出的无效根瘤突变体植株的生物量及固氮能进行测定,并对无效根瘤进行电镜切片观察,为今后进一步阐明固氮机制奠定基础.
HTML
-
本研究所用材料为No.1006,No.486,No.2568等3种无效根瘤突变体.这些突变体筛选自东京大学大学院农学生命科学研究科植物功能研究室用EMS处理野生型百脉根MG-20种子而获得的百脉根突变体库.
-
在TY培养基(5 g Triptone,3 g Yeast extract和0.83 g NaCl溶于1 L蒸馏水中)上,28 ℃下培养Mesorhizobium loti MAFF303099根瘤菌2 d后,用pH值为6.8的B&D(Broughton and Dilworth)液体培养基[19]稀释根瘤菌,再接种在野生和突变体百脉根植株上.
-
No.1006,No.486和No.2568无效根瘤突变体的种子,播种在赤玉土(鹿沼兴产)和Kreha soil(Kreha,Tokyo,Japan)(每公斤土壤中含有53 mg的NH3+和10 mg的NO3-)按14:1配制的含少量N源的土壤中,上面覆盖蛭石[20].
-
接菌10,20,30,40和50 d后,野生株和突变体各取10株样品植株,放入20 mL的玻璃管中,用盖封口;再以注入方式用乙炔替换玻璃管中10%的空气,在37 ℃条件下放置2 h后,用GC-17A Gas-Chromatogaph测定固氮量,即固氮能力.
-
野生株根瘤和无效根瘤,采用石蜡法切片后,再进行前固定处理(固定液组成:戊二醛2.5%,多聚甲醛2%,pH值为7.0,HEPES buffer 50 mmol/L,D.W 9.3 mL总量为20 mL;甲苯胺蓝染色液:甲苯胺蓝1%+硼酸钠1%)和后固定处理(固定液组成:四氧化锇2%,pH值为7.0,HEPES buffer 50 mmol/L,D.W 0.5 mL总量为2 mL)及树脂固定处理[21],最后在电子显微镜(日本电子制JEM-1400)下观察根瘤菌感染后对宿主细胞的破坏程度.
1.1. 材料
1.2. 方法
1.1.1. 根瘤菌培养和接菌
1.1.2. 栽培条件
1.1.3. 固氮能测定
1.1.4. 根瘤电镜观察
-
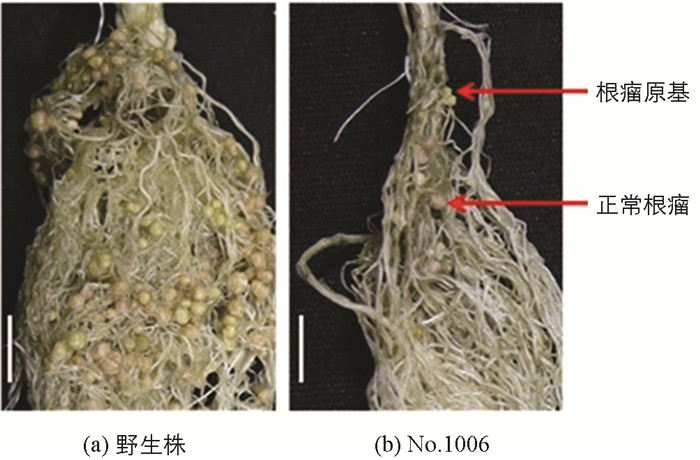
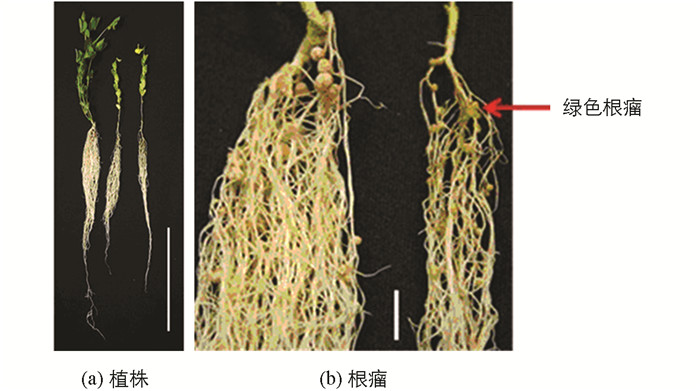
无效根瘤突变体接种根瘤菌后,与野生株对比观察突变体的根瘤形状及根瘤颜色.结果表明,No.1006突变体属于根瘤原基突变体,绝大多数根瘤原基凸起后停止、不形成正常根瘤,仅极少数发育形成正常根瘤(图 1);No.486突变体是结白色根瘤的突变体(发育成的根瘤呈白色)(图 2);No.2568突变体是结绿色根瘤突变体(发育成的根瘤呈绿色)(图 3).
-
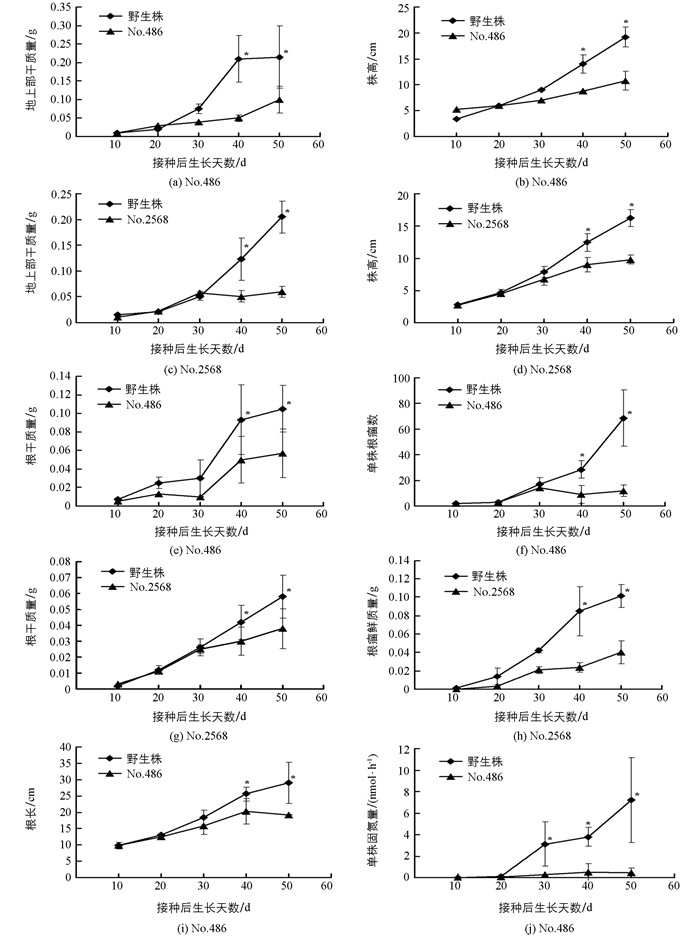
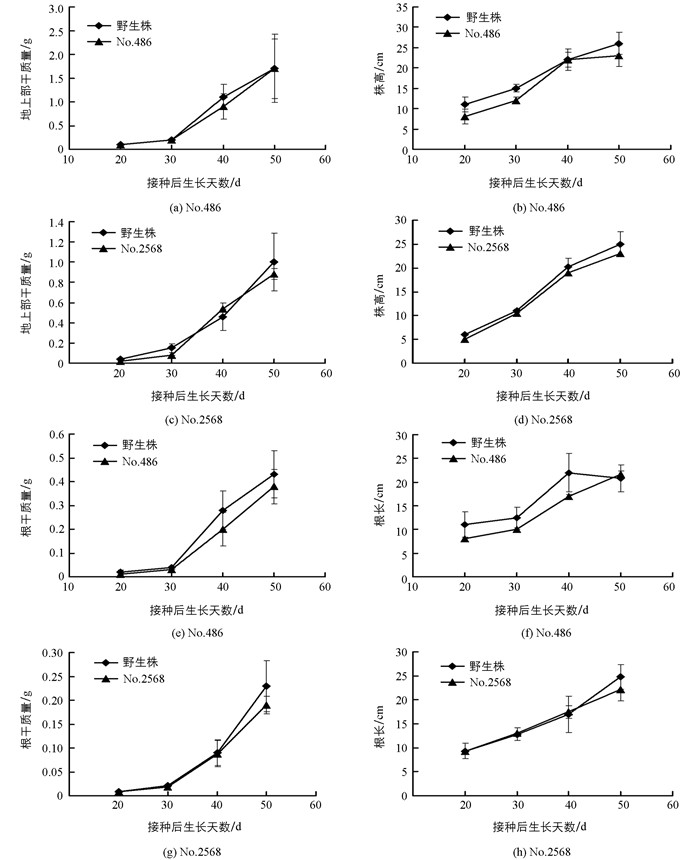
在含少量氮源的低氮基质(赤玉土:Kreha=14:1)中,无效根瘤突变体No.486和No.2568植株生长至30 d,其株高、地上部干质量、根干质量、根长及根瘤数等与野生株差异无统计学意义(p>0.05),但生长至40 d以后,各性状均显著小于野生株(p<0.05)(图 4);而在富氮基质(Kreha soil)中,无效根瘤突变体No.486和No.2568植株的株高、地上部干质量、根干质量和根长与野生植株差异均无统计学意义(p>0.05)(图 5).由于No.486和No.2568无效根瘤突变体植株所形成的根瘤数很少、远远少于野生株,固氮能力严重降低、无法或难以利用空气中存在的氮源,低氮基质中仅存在少量的氮时,无法满足植株生长所需,其植株的生长受到严重影响;而野生株由于正常形成根瘤、具备正常的固氮能力,在仅存在少量氮的低氮基质中通过固氮摄取其植株生长所需的氮,植株依然能够正常生长(图 4).而在富氮基质中,虽然No.486和No.2568无效根瘤突变体植株无法通过固氮摄取其植株生长所需的氮,但能够通过根系吸收基质中存在的氮、其根系摄取氮的能力并未受到影响,无效根瘤突变体植株生长与野生株相比差异无统计学意义(图 5),意味着这两种突变体的突变基因只是与根瘤的形成有关.
-
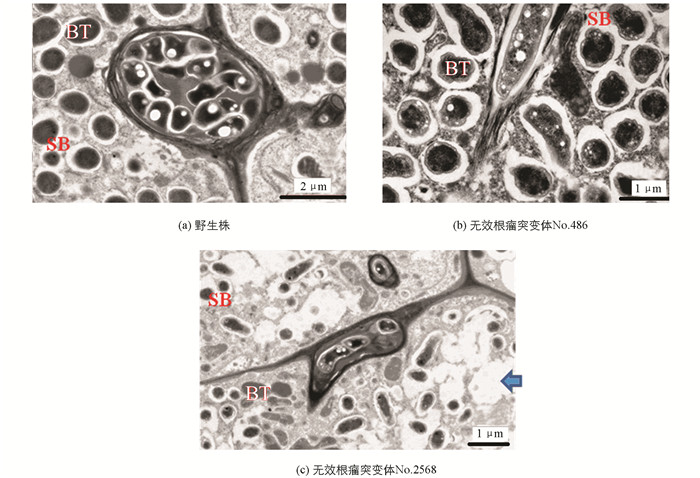
由于突变体No.1006是一种根瘤原基突变,故只对No.486无效根瘤突变体的白色根瘤和No.2568无效根瘤突变体的绿色根瘤进行了电子显微镜观察.结果显示,与野生百脉根根瘤(图 6a)相比,根瘤菌侵入后,这两种无效根瘤突变体的根瘤细胞,均有不同程度的破坏;其中,No.486无效根瘤突变体的白色根瘤细胞被破坏程度较轻(图 6b),而No.2568无效根瘤突变体的绿色根瘤细胞被破坏的程度则非常严重(图 6c).
-
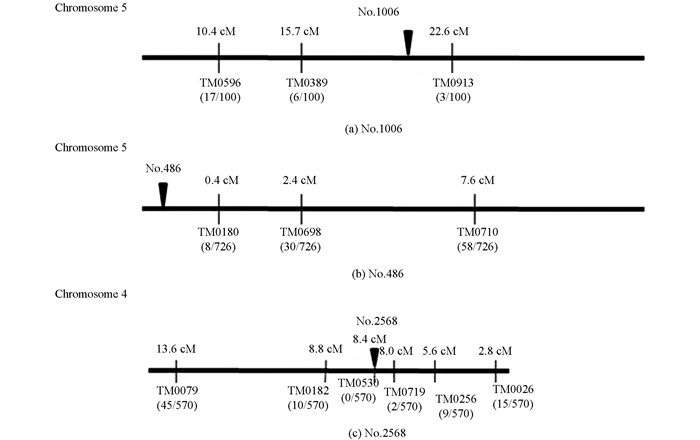
为确定No.1006,No.486和No.2568无效根瘤突变体的突变基因在百脉根染色体上的位置,这3种突变体与野生株杂交获得F1,经套袋自交获得F2群体;随机选取F2植株100株,苗期采集叶片,采用CTAB法提取DNA;利用92个SSR标记和dCAPS标记(http://www.kazusa.or.jp)进行基因分型,对无效根瘤突变体的突变基因进行了定位(图 7).结果表明,No.1006突变体的突变基因位于第5染色体上分子标记TM0389(15.7 cM)和TM0913(22.6 cM)之间(图 7a),标记间隔小于7 cM. No.486突变体的突变基因位于第5染色体顶端(图 7b),与邻近分子标记TM0180之间距离为0.4 cM.而No.2568突变体的突变基因位于第4染色体上分子标记TM0719(8.0 cM)和TM0530(8.4 cM)之间(图 7c),标记间隔小于0.5 cM.
2.1. 无效根瘤突变体的根瘤
2.2. 无效根瘤突变体植株的生物量、固氮能及根瘤数
2.3. 无效根瘤突变体根瘤细胞的电镜观察
2.4. 3种无效根瘤突变体的突变基因在百脉根染色体上位置
-
迄今为止,前人在多种豆科植物中筛选出许多无效根瘤突变体,这些无效根瘤突变体形成的无效根瘤与早期老化有关[10-11, 14, 22-23].这种早衰现象在百脉根(Lotus japonicus)被R.etliCE3根瘤菌感染形成的根瘤中也被观察到[12-17],但根瘤的早衰并非简单地与只形成无效根瘤进而造成无固氮能力有关[24];而且,与固氮有关的不同基因具有不同的功能,这些基因突变产生不同类型的突变体、固氮能力丧失机制也不同.
本研究中,No.1006突变体的突变基因被定位在第5染色体上分子标记TM0389和TM0913之间,该定位区间内存在的sym7基因突变所产生的表型[25],与本研究中No.1006突变体极为相似. No.486突变体的突变基因被定位在第5染色体顶端至接近顶端处分子标记TM0180之间,该定位区间内存在的ign1基因突变所产生的表型[26],与本研究中No.486突变体极为相似.而No.2568突变体的突变基因位于第4染色体上分子标记TM0719和TM0530之间,该定位区间内存在的sen1基因突变所产生的表型[7],与本研究中No.2568突变体极为相似.由于这些突变在百脉根染色体上的定位区间小,尤其是No.486和No.2568无效根瘤突变体的突变基因在百脉根染色体上的定位区间小于0.5 cM.所以,可以推断No.1006突变体的突变基因可能为sym7基因,No.486突变体的突变基因可能为ign1基因,而No.2568突变体的突变基因可能为sen1基因.
No.486无效根瘤突变体的白色根瘤和No.2568无效根瘤突变体的绿色根瘤组织的电镜观察结果显示,与野生百脉根根瘤相比,根瘤菌侵入后,这两种无效根瘤突变体的根瘤细胞均有不同程度的破坏,可能意味着ign1基因(No.486突变体的突变基因)和sen1基因(No.2568突变体的突变基因)的突变阻碍了根瘤菌与根细胞之间建立的共生体系;相对而言,sen1基因的突变对根瘤菌与根细胞之间共生体系建立的影响比ign1基因的突变更严重.据研究,ign1是编码类菌体和共生体的持续分化所必需的一种蛋白,拥有跨膜结构域和7个锚蛋白重复序列(Ankyrin-repeat),在共生体系中发挥着很重要的作用[26]. Sen1基因的突变[7],导致固氮酶活性完全丧失,进而造成类菌体(bacteroide)的根瘤菌分化能力丧失,引起感染细胞产生空胞、共生体的扩大等早期老化现象,最终导致形成无效根瘤[27-28].
总之,本研究中No.486突变体(推定为ign1突变)和No.2568突变体(推定为sen1突变)形成白色或绿色根瘤,其固氮能力显著低于野生株形成的红色根瘤;这两种突变体,导致根瘤菌侵入根细胞后在两者间共生体系的建立过程受到影响、根瘤菌不能转化为有功能的类菌体、无法形成有固氮能力的根瘤,最终导致严重降低或失去固氮能力.







 DownLoad:
DownLoad: