-
黄连(Coptis chinensis)是毛莨科多年生草本植物,有效药用成分为根部小檗碱[1],常用于治疗肠胃湿热、呕吐等疾病.我国黄连主要生长于湖北、贵州、重庆等地,连作障碍是限制黄连产量的重要原因,种植一轮的土壤通常需要休地5~10 a,这一现象也普遍存在于三七、太子参和地黄等药材种植中[2-3],造成该现象的原因包括土壤养分变化、酚酸类化感物质积累、土壤微生态失衡等[4].植物根系不断分泌化感物质到土壤中,高浓度的酚酸类化感物质破坏细胞膜功能[5],限制幼苗生长,抑制种子萌发所需的关键酶[6],其中阿魏酸(Ferulic acid)造成植株体内保护酶活性急剧下降进而强烈抑制幼苗根的生长.黄连在种植过程中产生的对羟基苯甲酸、香草酸、阿魏酸等酚酸类化感物质随种植年限增加而不断积累[7],这些化感物质的积累是导致黄连连作障碍的重要原因.
鉴于阿魏酸是黄连种植过程中大量积累的酚酸类物质,对幼苗根的生长的严重影响,作者从重庆市石柱县黄连种植土壤中筛选得到10株可以分解利用阿魏酸的真菌,挑选降解效果最好的菌株进行鉴定和降解特性测试,并模拟土壤环境检测对阿魏酸的降解效果,以期丰富黄连土壤来源的阿魏酸降解菌资源,为修复黄连连作障碍奠定一定的基础.
HTML
-
黄连根际土壤样品采自重庆石柱县黄连种植基地,无菌袋带回实验室当日处理.去除杂质后以无菌水梯度稀释,均匀涂板于加入25 mg/L氯霉素的PDA培养基上,于25 ℃培养至形成可见菌落后转接至无抗培养基中纯化培养.
-
将真菌制成有效菌数为1×107 cfu/mL的孢子悬液. 50 mL无机盐培养基中阿魏酸添加量为200 μg/mL,孢子悬液接种量为1%,28 ℃下培养72 h.收集菌体烘至恒重,计菌体质量增加量.取发酵液用等体积乙酸乙酯萃取后蒸干回溶于甲醇,高效液相检测阿魏酸质量浓度.色谱条件:分离柱为Waters公司的symmetey C18柱,检测波长为280 nm,柱温30 ℃,进样量为20 μL,流动相组分为甲醇和水(冰醋酸pH值调节至2.8),体积流量为1 mL/min.梯度洗脱设置:在12 min时,甲醇和水的体积比为40:60,其他时间(0,0.1,20 min)二者的体积比为30:70.
-
以SDS法裂解菌体提取基因组作为模板,真菌ITS通用引物(27F:AGAGTTTGATCMT GGCTCAG/1525R:AAGGAGGTGWTCCARCC)进行PCR反应. PCR反应条件如下:95 ℃预变性5 min,94 ℃变性30 s,55 ℃退火30 s,72 ℃延伸45 s,共30个循环.扩增所得片段电泳检测后送华大基因公司测序,所得菌株F-0056的ITS序列使用软件MEGA7.0进行系统发育分析,并提交至GenBank,获得的登录号为MH817153.
-
50 mL无机盐培养基中阿魏酸添加量分别为200,400,600,800和1 000 μg/mL,孢子悬液接种量为1%,在28 ℃条件下180 r/min转速培养72 h后HPLC检测阿魏酸质量浓度;50 mL无机盐培养基中阿魏酸添加量为200 μg/mL,孢子悬液接种量同样为1%,28 ℃条件下180 r/min转速培养,分别于24,48,72和96 h时HPLC检测阿魏酸质量浓度.
-
挖取黄壤土适量,将有效菌数为1×107 cfu/mL的F-0056孢子悬液以10:1的比列接种至1 kg的土壤中,以添加等量无菌水为对照,外源阿魏酸添加量为1 mg/mL,室温条件下存放.每隔6 d取每组土壤各50 g,以150 mL乙酸乙酯萃取,HPLC检测阿魏酸质量分数变化.
1.1. 样品采集和微生物的分离纯化
1.2. 酚酸降解菌筛选
1.3. 基因组提取和ITS鉴定
1.4. 阿魏酸的降解特性测试
1.5. 土壤环境下F-0056对阿魏酸的降解效果
-
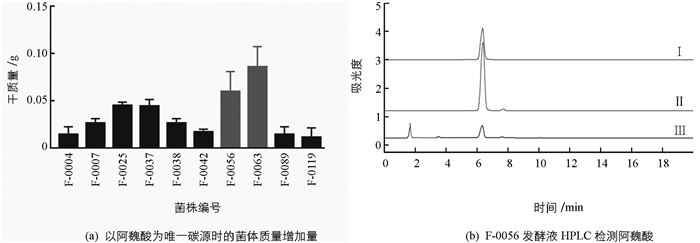
从黄连根际土壤中分离纯化得到真菌46株,接种至以阿魏酸为唯一碳源的培养基中72 h后,根据菌体质量增加量初步得到10株可在阿魏酸为唯一碳源的培养基中生长的真菌,表明它们可能有分解利用阿魏酸的能力,其中菌株编号为F-0056和F-0063的菌体质量增加最明显,菌株质量增加情况见图 1a. HPLC检测菌株F-0056发酵液中阿魏酸残留量(图 1b),发现培养72 h后F-0056几乎完全降解了阿魏酸,表明该菌具有高效降解阿魏酸的能力.
-
提取F-0056菌株基因组DNA,扩增ITS片段并测序比对,发现该菌株的ITS序列与图 2a中所示3株齿孢青霉ITS序列相似度达99%,故初步鉴定为齿孢青霉(Penicillium daleae).固体PDA培养基上培养2 d时F-0056菌落为白色丝绒状,第4 d时开始产绿色孢子,菌落形态见图 2b;刮取适量菌丝和孢子镜检可见由具横隔的分枝菌丝构成的营养菌丝体,并分化出青霉所特有的直立分生孢子梗,顶端可见成串孢子链(图 2c).
-
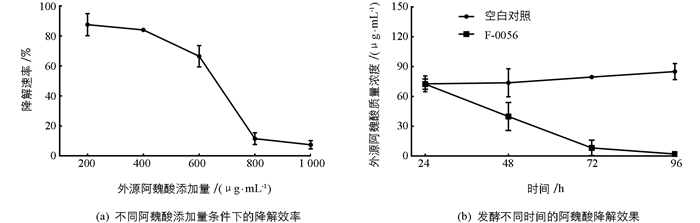
检测菌株F-0056培养72 h后阿魏酸的残留量,发现外源阿魏酸添加量为200 μg/mL时降解效率最高,降解率达87.5%(图 3a).随着外源阿魏酸添加量的增加,降解效果逐渐下降,当阿魏酸添加量增加至800 μg/mL时降解效率降至11.5%.等量外源阿魏酸条件下发酵24,48,72和96 h时阿魏酸降解效率见图 3b,相对于加入无菌水的空白对照而言,加入F-0056孢子悬液后阿魏酸的降解率随时间呈增长趋势,96 h时降解率达98.9%.
-
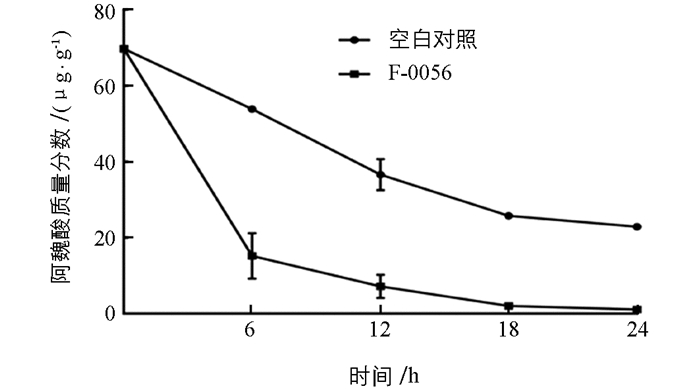
为了测试F-0056在土壤环境中的降解效果,模拟土壤条件定时检测该菌株对土壤中阿魏酸的分解(图 4),发现未加入孢子悬液的对照组土壤中阿魏酸质量分数在前18 d有缓慢下降趋势,而土壤中加入F-0056孢子悬液后对阿魏酸降解效果明显.第6 d时加入该菌的土壤中阿魏酸降解率达71.8%,明显高于未加入降解菌的土壤中阿魏酸的降解速率,24 d时土壤中阿魏酸几乎全部降解.
2.1. 阿魏酸降解真菌的筛选
2.2. 菌株F-0056的鉴定
2.3. 菌株F-0056对液体培养基中阿魏酸的降解特性
2.4. 菌株F-0056在土壤环境中对阿魏酸的降解效果
-
根茎类药材种植过程中普遍存在连作障碍,严重限制中药材的产量及品质.黄连在种植过程中产生的酚酸类化感物质对黄连幼苗的生长有抑制作用,其中以香草酸、4-香豆酸、阿魏酸对黄连生长的影响最为显著[8-9].阿魏酸是土壤中主要存在的酚酸类物质,会造成植株体内保护酶活性急剧下降而抑制幼苗根系生长,其他种类酚酸则干扰植物的光合作用、抑制植物对矿质营养元素的吸收,从而影响植株的正常生长[10-11].利用微生物降解环境中的有害物质,作为一项高效、环保的技术而受到人们的青睐[12-13],如土壤中筛到3株降解西瓜根系分泌物的放线菌,能降解土壤中阿魏酸的同时也是甜瓜枯萎病的拮抗菌[14];AM真菌是土壤生态系统中植物根系的互惠共生体,能够有效缓解连作障碍[15];黄孢毛平革菌在300 mg/L阿魏酸培养基中降解效率为99% [16].作者从不同年份的黄连种植土壤中筛选得到10株能够分解利用阿魏酸的真菌,其中一株齿孢青霉对阿魏酸具有高效降解能力,培养4 d的降解效率高达98. 9%.更为重要的是,该菌能够在盆栽土壤中快速、有效地分解阿魏酸,表明该菌可能用于黄连连作土壤中阿魏酸的降解.此外,齿孢青霉对立枯病菌、锈腐病菌和黑斑病菌等植物病原菌有抑菌效果[17],因此该菌株除了可以用于缓解土壤中阿魏酸积累造成的连作障碍,还可能具有潜在的生防效果,这需要进一步研究.本研究从黄连土壤中分离得到多株阿魏酸降解菌,丰富了土壤来源的阿魏酸降解菌资源,为快速修复化感物质积累造成的中草药连作障碍奠定了一定的基础.







 DownLoad:
DownLoad: