-
精神分裂症是一种病因尚未明确的常见重性精神疾病,在世界人口中的患病率大约为1%.目前常用的抗精神病药物(Antipsychotic drugs,APDs)使患者面临不同程度的肥胖、高血压、高脂血症和高胰岛素血症的危险.然而,导致这些不良反应的机制尚不明确[1].
氟哌啶醇,第一代抗精神病药物(First-generation antipsychotic drugs,FAGs)的代表,有较强的多巴胺受体拮抗作用,较其他APDs有着更为明显的不良反应,如锥体外系反应较重、过度镇静和体质量增加[2].利培酮作为目前临床上常用的二代抗精神病药物(Second-generation antipsychotic drugs,SAGs),对中枢系统的5-羟色胺和多巴胺拮抗作用有平衡作用,可以减少锥体外系反应的发生.然而,这类药物的广泛应用使血糖、血脂等代谢紊乱的不良反应凸显,引起了学者的关注[3].阿立哌唑是继FAGs和SAGs之后的,对精神障碍治疗具有革新性突破的新一代药物,又称为“第三代抗精神病药物”[4].它是一种喹啉衍生物,具有独特的作用机制——多巴胺系统稳定剂[5],对H1受体和M受体有轻到中度的阻断作用,出现体质量增加,嗜睡的可能性减小,从而很少引起体质量增加、血脂及血糖的升高,甚至对血糖、血脂有轻微的降低作用[6].
之前,人们认为,APDs介导肥胖主要源于药物作用于中枢神经系统后,改变了摄食、自发活动以及体温等间接性作用导致,而糖脂代谢紊乱可能是肥胖的后续反应[7-8].近年来,有研究陆续发现APDs介导的代谢紊乱完全可能独立于体质量,药物则通过影响外周肝脏等器官中脂代谢相关基因的表达介导这类副反应的发生[9-10].三代药物对多巴胺、五羟色胺等受体具有不同的亲和效应,对外周组织中脂代谢相关基因的表达是否也产生了不同的影响,从而介导不同的代谢性副反应呢?为了进一步比较他们的安全性,并探究他们在短期作用下引起代谢紊乱的可能机制,本研究通过氟哌啶醇、利培酮和阿立哌唑短期作用,对小鼠体质量、摄食、糖脂代谢参数以及行为学变化进行分析,并检测肝脏组织中脂代谢相关的胆固醇调节元件结合蛋白(Sterol-regulatory element binding proteins,SREBPs)和组胺受体(Histamine 1 receptor,H1R)的表达水平,为APDs介导代谢性副反应的机制及干预研究提供思路.
HTML
-
雄性昆明小鼠,体质量20~22 g.采用IVC独立送风隔离笼具及标准饲料饲养于室温为20~24 ℃,相对湿度为60%左右的SPF级动物室中,每笼2只.自由饮水进食,使其适应环境1周.
-
利培酮(RIS)、氟哌啶醇(HAL)、阿立哌唑(ARI)和MK801均为固体粉末,由美仑生物提供;实验时,利培酮、氟哌啶醇、阿立哌唑用5%吐温80悬浮,MK801用生理盐水溶解,根据实验需要配制成不同浓度备用.葡萄糖测定试剂盒,上海荣盛生物药业有限公司;总胆固醇测定试剂盒和甘油三酯测定试剂盒,浙江东瓯诊断产品有限公司;RIPA裂解液及BCA蛋白测定试剂盒,北京鼎国昌盛生物技术有限责任公司.
-
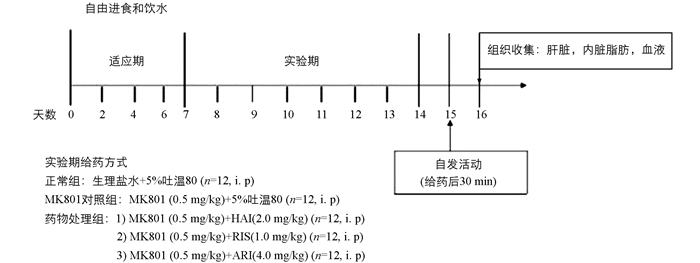
挑选生长状况良好的小鼠60只,将其随机分为5个组,每组12只,称质量并记录.第1组为正常组,第2组为MK801对照组,后3组为药物处理组. 8:00和20:00以腹腔注射方式(i.p)分别给予正常组生理盐水,MK801对照组5%吐温80溶液(所有药物溶剂),药物处理组氟哌啶醇(2.0 mg/kg)、利培酮(1.0 mg/kg)或阿立哌唑(4.0 mg/kg),并于早上给药结束后30 min,腹腔注射MK801(0.5 mg/kg),见图 1.所有给药量均以小鼠体质量计算所得.
-
实验开始前对小鼠进行体质量测定并记录,给药期间隔天记录小鼠体质量和摄食量,直至行为学实验开始.
-
在连续给药7 d后,于第8 d进行自发活动测定.在早上给药30 min后,将小鼠放入黑色测试箱中,视频摄像机从测试箱上方记录小鼠30 min的行为.记录结束后用75%酒精对测试箱进行消毒除味.
-
连续给药9 d后,用5%水合氯醛麻醉小鼠,心脏采血至肝素钠抗凝管中,经500 g离心10 min后得血浆,放入-20 ℃冰箱,待分析.采血后,再进一步收集肝脏和内脏脂肪,分别记录质量(g),储存在-80 ℃冰箱下用于随后蛋白表达分析.
-
利用葡萄糖(G)测定试剂盒、甘油三酯(TG)测定试剂盒、总胆固醇(TC)测定试剂盒分别检测血浆中G,TG,TC浓度.所有的实验测定均按照制造商的说明,平行3次完成.
-
使用添加有蛋白酶和磷酸盐抑制剂混合物的RIPA缓冲液提取肝脏蛋白质样品.通过BCA蛋白测定法定量蛋白质浓度.所有样品加入上样缓冲液后在100 ℃下煮沸10 min.通过SDS-PAGE分离蛋白质,并转移至PVDF膜[11].使用以下一抗进行免疫印迹:Anti-SREBP1 antibody(ab3259);Anti-SREBP2 antibody(ab30682);Histamine H1 Receptor(sc-20633);β-actin Polyclonal antibody(20536-1-AP).二抗为辣根过氧化物酶缀合的抗体(Santa Cruz Biotechnology,Inc,sc2004和sc2005),并通过化学发光(ECL)试剂(Bio-Rad Laboratories,lnc)来接收检测荧光信号.使用Quantity One图像分析软件量化信号.将SREBP-1,SREBP-2和H1R与β-actin的相对蛋白表达用正常组的表达水平标准化.
-
所有数据均为x(平均值)±s(标准差),并由SPSS 19.0软件进行分析:体质量累积增加和食物累积摄入量采用双因素方差分析(时间与药物处理);自发活动、血糖血脂采用单因素方差分析;Dunnett-T test对组间作两两比较;非参数检验Mann-Whitney U test用于蛋白表达分析;Pearson's检测用于参数间相关性分析.当p<0.05或p<0.01时差异有统计学意义.
1.1. 实验材料
1.1.1. 实验动物
1.1.2. 实验试剂
1.2. 实验方法
1.2.1. 给药方式
1.2.2. 体质量及摄食量测定
1.2.3. 小鼠自发活动(Locomotion)的测定
1.2.4. 血浆、肝脏及内脏脂肪的收集
1.2.5. 血糖血脂检测
1.2.6. 蛋白免疫印迹分析
1.3. 统计学分析
-
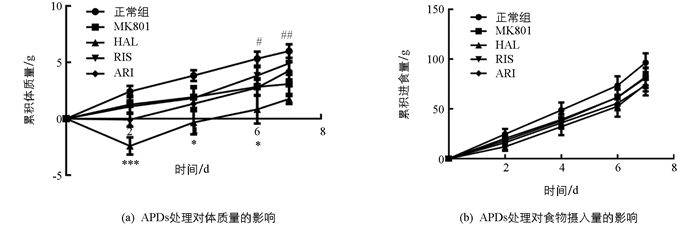
双因素方差分析显示(时间与药物处理),随着时间的增长(F(4,44)=43.29;p<0.01)及药物处理(F(4,44)=8.100;p<0.01)的介入,小鼠体质量发生了显著性变化.模型组小鼠相比于正常组,体质量显著降低(F(1,11)=7.364;p<0.05),尤其是在MK801处理后第6 d和第7 d(均为p<0.05).与模型组比较,APDs作用也引起了体质量的显著变化(F(3,33)=4.472;p<0.01),尤其是第2,4,6 d,HAL介导了小鼠体质量的显著降低(p<0.05).但RIS和ARI作用后,比较模型组小鼠均未出现显著的体质量变化.处理时间与药物作用之间交互作用无统计学意义(F(12,132)=1.697;p=0.0743)(图 2a).在摄食方面,模型组与正常组小鼠差异无统计学意义(F(1,11)=3.678;p=0.1135).而APDs处理组与模型组比较,摄食量虽然有显著的时间效应(F(4,44)=71.98;p<0.01),但组间差异无统计学意义(图 2b).
-
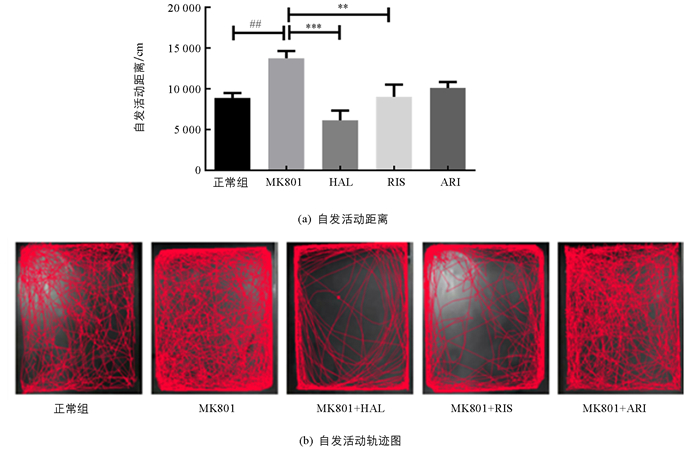
为了探索药物介导的代谢性副反应与镇静作用的相关性,我们检测了小鼠的自发活动(图 3).药物引起了小鼠自发活动的显著变化(F(4,55)=6.897;p<0.01).与正常组相比,模型组小鼠自发活动显著增加(p<0.05);与模型组相比,HAL(p<0.001)和RIS(p<0.01)显著降低了小鼠的运动距离,而ARI虽然在一定程度上也减少了MK801诱导的焦虑样症状,但没有显著性效应.
-
药物处理结束后,我们对各药物处理组的小鼠肝脏、内脏脂肪以及体脂比进行了分析.结果如表 1,显示短期药物处理对小鼠肝脏及内脏脂肪蓄积并不产生显著影响.
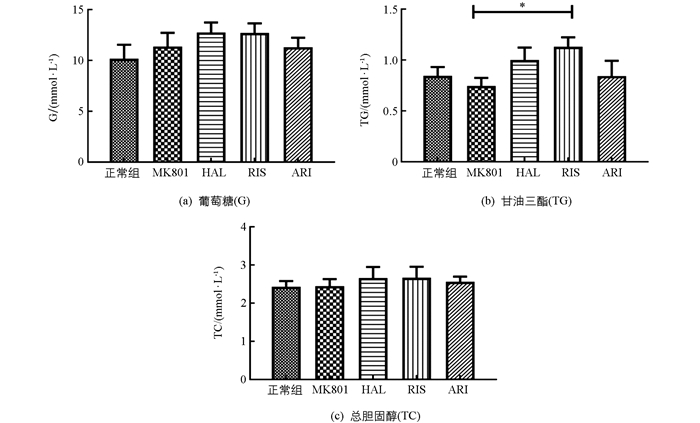
随后,我们分析了小鼠血糖血脂等生化参数.药物并未引起血糖(G)和总胆固醇(TC)的异常变化,但较强地促进了甘油三酯(TG)浓度的增加(F(4,54)=2.066;p=0.098).模型组小鼠血浆中G,TG和TC浓度与正常组相比,差异无统计学意义;但利培酮(RIS)显著增加了MK801模型鼠中血浆中TG的浓度(p<0.05),而其他两种药物作用后,小鼠血糖、血脂均无显著变化(图 4).
-
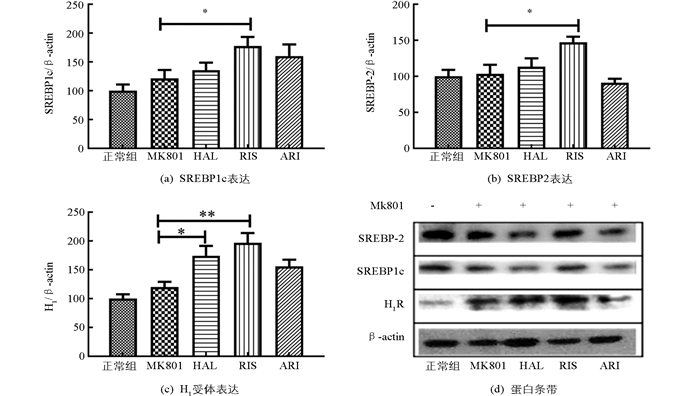
为了进一步观察药物对肝脏中脂肪代谢相关蛋白的影响,分析了两个重要的转录因子SREBP1c和SREBP-2以及组胺H1受体(H1R).结果显示,MK801处理并未引起小鼠肝脏中SREBP1c,SREBP-2,H1R蛋白表达量的显著性变化;仅利培酮(RIS)的短期作用,显著增加了MK801模型鼠中肝脏SREBP1c,SREBP-2和H1R的表达量(p<0.05),氟哌啶醇(HAL)也显著增加H1R蛋白的表达(p<0.05),见图 5.
-
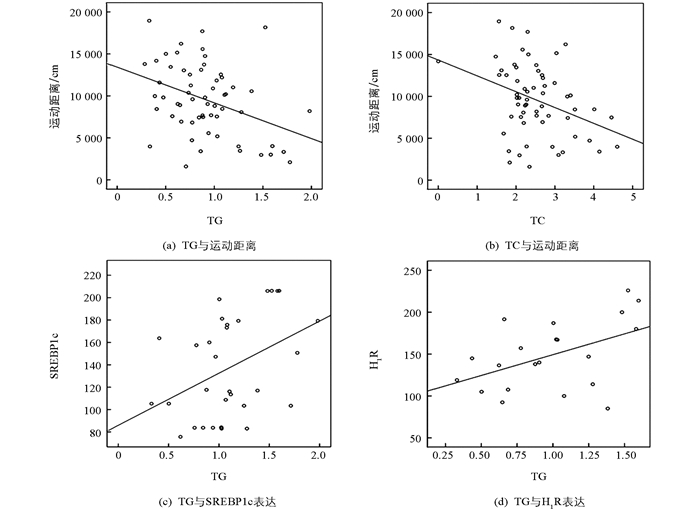
利用Pearson's检测,进行参数间相关性分析.结果显示,小鼠的体质量增加与其摄食和运动距离没有显著的相关性.而血浆中TG和TC浓度与自发活动中运动距离呈显著的负相关(r=-0.379,p<0.01,和r=-0.354,p<0.01);此外,TG浓度还与肝脏内SREBP1c(r=0.408,p<0.05)和H1R(r=0.484,p<0.05)蛋白表达呈显著正相关(图 6).
2.1. 药物对精神分裂症模型鼠体质量及摄食的影响
2.2. 药物对精神分裂症模型鼠自发活动的影响
2.3. 药物对精神分裂症模型鼠肝脏、内脏白色脂肪及血糖血脂的影响
2.4. 药物对精神分裂症模型鼠肝脏中SREBP和H1R的影响
2.5. 相关性分析
-
氟哌啶醇、利培酮和阿立哌唑为临床治疗重型精神疾病的常用药物.本研究通过对小鼠做短期药物处理,考察了3种抗精神分裂症药物对精神分裂症模型鼠多项代谢指标、行为学及脂肪代谢相关蛋白的影响,借此初步判断这类药物引起代谢性副反应的机制.
在以往关于APDs引发代谢性副反应的研究中,大多数采用正常成年鼠[12-13].但多项临床研究表明,首发精神分裂症患者在未服用抗精神病药物之前已经存在糖、脂代谢紊乱[14-15].为了更好地考察APDs对自发活动的影响是否为介导代谢性副反应的重要因素,实验中,我们采用多次注射NMDA受体拮抗剂MK801的方式,建立模拟精神分裂症高自发活动性的小鼠模型. NMDA受体拮抗剂是目前在前临床研究中最常用的精神分裂症动物模型,能够在动物和正常人中诱导出与临床精神分裂症非常相似的表型.大部分研究均显示,MK801可引起啮齿科动物自发行为增多[16-18].本研究结果也显示,模型组动物的自发活动(焦虑、快速移动)较正常组出现了显著变化,成功诱导小鼠产生了类似精神分裂症症状效应.同时,MK801诱导的精神分裂症小鼠较对照组的体质量有显著的减低.相关性分析显示,正常组与模型组小鼠体质量与自发活动距离之间存在显著的相关性(r=-0.464,p<0.05),说明MK801引起的显著自发活动的增加是模型组体质量降低的重要原因.而APDs介入治疗,促使了体质量的进一步减少,尤其是HAL,引起了该组小鼠体质量的显著下降.虽然,在临床上,HAL的使用并未介导显著的体质量变化,但我们的实验结果与之前采用雄性C57BL/6小鼠的研究结果一致[19].其中的原因可能是HAL引起的过度镇静,导致动物摄食减少,体质量增加减慢.
短期的药物处理,血脂中的TG,TC的浓度,与动物自发活动的距离呈显著的负相关(分别为r=-0.379,p<0.01;r=-0.354,p<0.01);而TG的浓度还与肝脏中重要的转录因子SREBP1c的表达增加呈显著的正相关(r=0.408,p<0.05),与组蛋白受体H1R表达的增加也呈显著的正相关(r=0.484,p<0.05).尤其是利培酮,表现出较强的引发代谢紊乱的趋势,促进了肝脏脂肪合成基因重要转录因子SREBP1c和SREBP-2,以及重要的组胺受体蛋白H1R的表达显著升高.肝脏是机体脂肪代谢最主要的外周组织,负责体内脂肪酸、胆固醇、磷脂合成.合成后的TG,TC与载脂蛋白一起形成极低密度脂蛋白,分泌入血.甾醇调节因子结合蛋白SREBPs是一组重要的脂肪酸及胆固醇合成调控因子,在脂代谢途径中起核心作用,其主要家族成员包括SREBP1a,SREBP1c,SREBP-2[20]. SREBP1c和SREBP-2分别负责包括脂肪酸合成酶在内的脂肪酸合成基因和胆固醇合成转运基因的转录调控,过量的SREBP活动可能导致循环胆固醇、游离脂肪酸和三酰基甘油升高.多项研究表明氯氮平、奥氮平等可以直接增强外周组织中SREBP脂肪合成调控通路中基因的转录,从而引起血脂升高[21-24]. Lauressergues等采用腹腔注射的方式对成年雌性C57BL/6小鼠进行长期的利培酮处理,结果显示,药物能显著地促进体质量、肝脏以及内脏脂肪质量增加,而这些指标与肝脏内转录因子SREBP1c及其靶基因Fas的转录水平呈显著的正相关.而后,他们通过大鼠原代肝细胞模型检测利培酮对细胞的直接作用时,发现该药物能显著增加SREBP1c和SREBP-2的蛋白表达,并促进细胞内游离脂肪酸以及TG的聚集[9]. SREBPs作为脂质生物合成途径中的核心分子,在其发挥转录调控效应时往往是以4个蛋白复合体的形式存在,其中包括SREBPs自身蛋白、负责将无活性的SREBPs从内质网转运至高尔基体完成剪切加工的SREBP剪切活性蛋白(SCAP)、结合于SCAP上的胰岛素诱导基因INSIGs以及孕酮受体膜组分1(PGRMC1).在氯氮平或利培酮(非阿立哌唑和氟哌啶醇组)处理4周的SD大鼠模型中,类固醇拮抗剂——米非司酮(mifepristone)能逆转因APDs药物处理而减少的PGRMC1/INSIG表达,同时也促使表达上调的SCAP/SREBP蛋白回到正常水平,而其血脂水平也较APDs处理组显著下降[25].因此,肝脏中PGRMC1/INSIG/SCAP/SREBP途径极可能是APDs诱导脂代谢紊乱的物质基础,而该作用并非体质量过度增加引起的后续反应.虽然这类药物介导的脂代谢紊乱程度不完全相同,但该副反应完全可能独立于体质量,而通过影响中枢神经系统活性和外周肝脏等器官中脂代谢相关基因表达等两个方面而产生.
此外,本研究中,APDs作用后,肝脏中组胺H1受体表达量与血浆中TG浓度也呈现显著的正相关(r=0.484,p<0.05).事实上,APDs具有多靶点效应,除了对多巴胺D2受体(D2R)产生较强的拮抗作用来发挥正常的药理作用外,越来越多的研究认为药物与下丘脑系统中五羟色胺2A(5-HT2A)和2C(5-HT2c)受体、组胺H1受体以及乙酰胆碱能M3受体不同的亲和效应是其介导肥胖以及代谢紊乱的重要原因[26-27]. Lian等人采用组胺受体激动剂——倍他司汀与奥氮平共同处理SD大鼠,发现APDs对H1R的阻断可能导致下丘脑H1R密度的代偿性上调,由此激活AMP依赖的蛋白激酶(AMPK)通路,引起摄食增加从而导致肥胖[8, 28-30].与其他受体相比,APDs对H1的拮抗效应被认为是APDs诱导体质量增加的主要靶点[31]. APDs是否经肝脏上组胺受体,介导下游脂肪合成相关蛋白的合成上调,从而引发脂代谢紊乱,我们还需在今后的工作中进一步研究.
综上所述,短期作用下,阿立哌唑无明显糖脂代谢紊乱,具有安全性高、不良反应小的特点.氟哌啶醇等一代抗精神病药物,因其过度镇静效应,可能是这类药物诱发一定程度的代谢性副反应的主要原因;而利培酮作为临床治疗重型精神疾病的一线用药,能较好地发挥疗效,但因其与其他靶点(SREBPs,H1R)的亲和效应,可能引起更为严重的代谢紊乱副反应,并独立于体质量的增加.总之,重型精神疾病需要长期用药,在服药期间,做好及时有效的代谢监测不仅有利于增加患者的依从性,对其可能导致的严重心血管疾病也能更好地预防和干预.






 DownLoad:
DownLoad: