-
太子参(Radix pseudostellariae)为石竹科草本植物孩儿参的干燥块根,为常用补气中药,具有益气生津、补肺健脾之功效,其作用与人参相似[1].太子参块根中太子参须多糖含多种生物活性成分[2],如糖苷类、环肽类、氨基酸、皂甙、微量元素、磷酯类、脂肪酸类等[3].其中,多糖是太子参块根的主要活性成分,它在抗炎和调节机体免疫功能方面具有显著的作用[3].有研究显示,太子参多糖对运动诱导所致的氧化应激大鼠具有保护作用[4-5].
近年来,由于临床大量使用以及功能性食品的开发利用,太子参价格一路攀升.而太子参须为太子参在加工过程中被废弃的不定根和根尖部分,占太子参质量的10%~15%,其含丰富的多糖和皂苷等成分,被当废物丢弃,造成资源的大量浪费.因此,为减少浪费、充分且合理开发资源,本实验检测了太子参须多糖粗提物对RAW264.7巨噬细胞吞噬中性红及隐球菌(Cryptococcus neoformans)的影响,并在隐球菌感染小鼠模型上进一步研究了太子参须多糖粗提物对小鼠免疫功能的调节作用,检测肺部组织中Toll样受体(Toll-like receptors,TLR)和环磷腺苷效应元件结合蛋白(cAMP-response element binding protein,CREB)的表达水平,为太子参须的进一步开发利用提供药理学依据.
HTML
-
本实验所用太子参须来自贵州施秉,经刘汉儒及曾忠良老师鉴定为太子参须根及尾根,样本保存在西南大学药学院,编号SWU-R.P.-G-1.
-
小鼠巨噬细胞系RAW264.7为西南大学药学院实验室保存;新生隐球菌H99(Cryptococcus neoformans H99)为廖国建老师惠赠;昆明(Km)小鼠,SPF级,18~22 g,雌雄各半,购于陆军军医大学实验动物中心,动物质量合格证号:SCXK(渝) 2018-0003.
-
DMEM高糖培养基、四季青血清,浙江天杭生物科技股份有限公司;磷酸盐缓冲液(PBS)、青霉素-链霉素溶液,上海碧云天生物技术有限公司;二甲基亚砜(Dimethyl sulfoxide,DMSO),北京鼎国昌盛生物技术有限责任公司;四甲基偶氮唑盐(Methythia-zolyltetrazole,MTT)、中性红(Neutral Red),瑞士Adamas公司;小鼠补体(Mouse Complement),上海泰坦科技股份有限公司;YPD液体培养基:蛋白胨20 g/L,葡萄糖20 g/L,酵母浸出粉10 g/L,pH值为6.5±0.2.
白细胞介素-1β(interleukin1 beta,IL-1β)、肿瘤坏死因子-α (tumor necrosisfactor,TNF-α)、白细胞介素-6(interleukin 6,IL-6)、白细胞介素-10(interleukin 10,IL-10)酶联免疫吸附测定(enzyme-linked immunosor-bent assay,ELISA)试剂,杭州联科生物技术股份有限公司. Anti-CREB(Cell Signaling Technology,#9197),Anti-p-CREB(Cell Signaling Technology,#9198),Anti-TLR4(Bioss,bs-1021R),Anti-GAPDH(Bioss,bs-0755R)抗体及二抗(Bioss,bs-0311P-HRP)用于蛋白免疫印迹实验,并通过化学发光(ECL)试剂(Bio-Rad Laboratories,lnc)接收检测荧光信号.将CREB,p-CREB和TLR4与GAPDH的相对蛋白表达用正常组的表达水平标准化,并使用Image J图像分析软件量化信号.
1.1. 太子参须多糖制备
1.2. 菌株及实验动物
1.3. 实验试剂
-
太子参须→除杂→粉碎,80目过筛→80%乙醇回流→过滤丢弃乙醇→残渣加水回流提取→旋转蒸发浓缩→5倍体积的95%乙醇静置24 h→多糖沉淀→4 000 r/min离心15 min收集沉淀→无水乙醇洗涤→减压干燥→多糖粗品.由苯酚-硫酸法测定多糖质量浓度(C多糖)[6].
式中,C样品为样本质量浓度,R稀释为稀释倍数,V样品为样品体积,W多糖为多糖试样质量浓度.
-
RAW264.7巨噬细胞为一种来源于小鼠的无限增殖巨噬细胞系,采用DMEM高糖培养基(含10%胎牛血清,1%青霉素-链霉素溶液),于37 ℃,5% CO2培养箱中培养.取对数期RAW264.7巨噬细胞,铺板,分别设空白对照组(DMEM培养基)、对照组(不含太子参须多糖)、实验组(1,5,10,20,30,50 μg/mL等终浓度的太子参须多糖),于37 ℃,5% CO2培养箱中分别培养16和24 h.培养结束后,加入MTT溶液继续孵育4 h;弃培养液,加入DMSO,在490 nm处测定吸光度(OD),计算细胞增殖率(P).
-
细胞参照“2.2 ”项下方法培养,待细胞贴壁后,更换为含药培养基,给药方案如下:设置空白对照组,模型组[1 μg/mL的脂多糖(LPS)和50 U/mL的干扰素(INF)],太子参须多糖组(含1 μg/mL LPS和50 U/mL INF,根据MTT实验结果确定多糖终浓度为0.1,1,5,10 μg/mL). 37 ℃,5% CO2培养箱培养24 h.弃培养基,加入0.1%中性红溶液,共培养30 min.弃中性红,用PBS清洗,加入100 μL细胞裂解液(冰乙酸与无水乙醇比例为1:1),室温放置2 h,酶标仪测其490 nm处吸光度值(OD),计算细胞吞噬率(S).
-
细胞参照“2.2 ”项下方法培养,待细胞贴壁后,更换为含药培养基,具体给药方案如下:设置空白对照组,模型组(1 μg/mL LPS和50 U/mL INF),太子参须多糖组(1 μg/mL LPS和50 U/mL INF),根据MTT实验结果确定多糖终浓度为0.1,1,5,10 μg/mL). 37 ℃,5% CO2培养箱培养24 h,隐球菌在YPD液体培养基中培养48 h,收集菌体,PBS洗涤,调整菌体浓度为2×107个/mL.加入小鼠补体使终浓度为20%的隐球菌混悬液,室温放置10 min,取10 μL隐球菌小鼠补体共培养物加入24孔板中,将隐球菌与巨噬细胞共培养2 h后,在倒置显微镜下观察巨噬细胞对隐球菌的吞噬情况;PBS洗涤3次,将未被巨噬细胞吞噬的胞外隐球菌去除,无菌水裂解细胞10 min,释放被吞噬的胞内隐球菌,再分别稀释成100,1 000倍后,将其涂布在YPD固体培养基平板上,30 ℃培养48 h,计数.
-
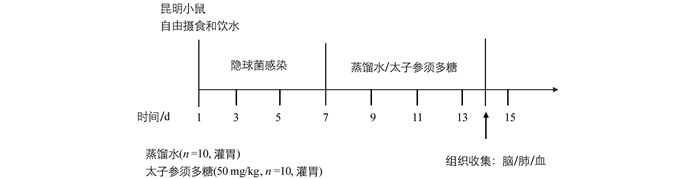
将Km小鼠随机分成空白对照组、隐球菌感染模型组、太子参须多糖治疗组,每组10只.给药方案如图 1,第1~2 d,对空白组小鼠作去离子水滴鼻处理,模型组及治疗组小鼠作新生隐球菌H99(2×106个/mL,50 μL/只)滴鼻处理.第7 d以后,空白组和模型组小鼠胃管灌服蒸馏水,治疗组小鼠灌服50 mg/(kg·d)太子参须多糖,连续7 d,期间各组均自由摄食和饮水.分别于实验前、实验第7,9,11,13 d对小鼠进行称质量,记录其体质量的变化和精神状态.末次给药后,称质量,麻醉小鼠后心脏采血,并收集肺、脑组织.收集血浆,用ELISA检测IL-1β,IL-6,IL-10及TNF-α炎症因子分泌量.肺及脑组织匀浆后,脑组织稀释10,100倍,肺组织稀释100,1 000倍,将其分别涂布在YPD固体培养基平板上,30 ℃培养48 h,计数.
-
操作严格按照试剂盒说明书进行:实验前将所有的试剂、样本平衡至室温;按300 μL/孔体积将1×洗液加入预包被酶标板,静置30 s,弃洗液,空白孔加入标准品稀释液,样本孔加入检测缓冲液和血浆样本,并在每孔加入对应的检测抗体.板封膜后室温振动孵育30 min.弃废液,加入洗液洗涤6次,再加入辣根过氧化物酶(HRP)标记的链霉亲和素.室温振动孵育30 min,洗液洗涤后加入显色剂,在450和570 nm处测吸光度值(OD).
-
使用添加有蛋白酶和磷酸酶抑制剂混合物的RIPA缓冲液提取肝脏蛋白质样品.通过BCA蛋白测定法测定蛋白质浓度.所有样品加入上样缓冲液后在100 ℃下煮沸10 min.通过SDS-PAGE分离蛋白质,并转移至PVDF膜[7]. 5%脱脂牛奶封闭2 h,加入一抗,4 ℃孵育过夜,TBST漂洗3次,每次15 min;再加入相对应二抗室温孵育2 h;TBST洗涤后,ECL试剂盒曝光成像,Quantity one软件对得到的灰度值进行分析.
-
实验结果均采用平均值±标准差(M±SD),结果分析利用SPSS 19.0作单因素方差分析,计算p值,当p<0.05时,差异有统计学意义.
2.1. 太子参须多糖的提取及质量浓度测定
2.2. MTT法检测太子参须多糖对RAW264.7巨噬细胞增殖的影响
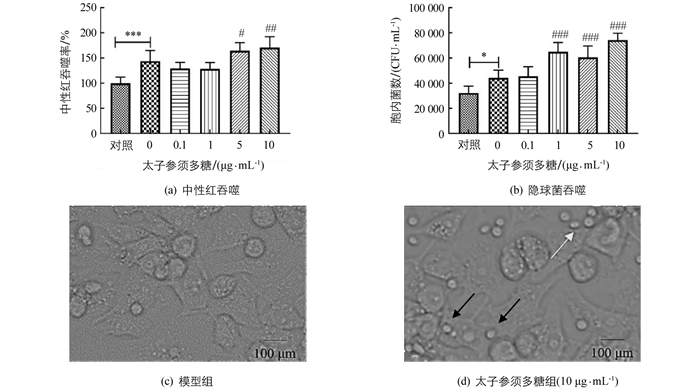
2.3. 太子参须多糖对巨噬细胞吞噬中性红能力的影响
2.4. 太子参须多糖对巨噬细胞吞噬隐球菌的影响
2.5. 实验动物分组与处理
2.6. 血浆中炎症因子的检测(ELISA法)
2.7. 蛋白免疫印迹分析
2.8. 统计学分析
-
依据葡萄糖标准溶液与对应的吸光度,绘制葡萄糖标准曲线(图 2),拟合线性方程为
根据方程计算多糖质量浓度为82.9 mg/mL.
-
如表 1,以对照组细胞在适宜条件下培养的增殖率为100%计,太子参须多糖处理RAW264.7巨噬细胞16与24 h后,与对照组相比,浓度为1 μg/mL~20 μg/mL时,显著增强巨噬细胞的相对增殖率(p<0.01).
-
用太子参须多糖处理24 h,RAW264.7巨噬细胞的吞噬作用明显增强(p<0.001).与空白对照组相比,模型组中巨噬细胞对中性红和隐球菌吞噬率显著高于空白对照组;与模型组相比,5 μg/mL和10 μg/mL太子参须多糖组对中性红和隐球菌吞噬率显著增强,且呈剂量依赖性:1,5,10 μg/mL太子参须多糖均能显著提高巨噬细胞对隐球菌的吞噬能力(图 3).由图 3c和3d可以看出,10 μg/mL太子参须多糖能募集隐球菌向巨噬细胞靠近,有利于巨噬细胞的吞噬.
-
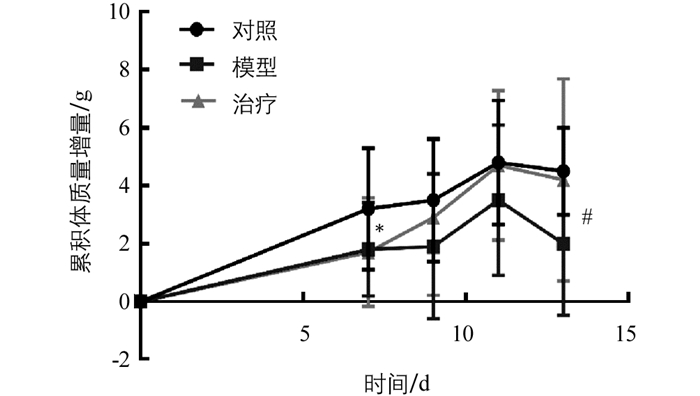
实验小鼠体质量的变化情况见图 4.用隐球菌感染小鼠7 d后,感染组相比于对照组体质量明显下降(p<0.05),连续一周每天给予灌胃(50 mg/kg)太子参须多糖后,治疗组与模型组相比,治疗组体质量显著高于模型组(p<0.05),且经过太子参须多糖治疗后小鼠体质量恢复到与对照组相近,说明太子参须多糖能明显增强隐球菌感染小鼠的免疫功能.
-
小鼠血浆中炎症因子的分泌量见图 5.太子参须多糖能显著抑制促炎因子IL-6的分泌量(p<0.001),并能显著提高抗炎因子IL-10的分泌量(p<0.05).部分增加IL-1β及TNF-α的分泌量,IL-1β及TNF-α的浓度越高,巨噬细胞和体液免疫的功能越强,小鼠的抗病能力越强.综合说明太子参须多糖对隐球菌感染的小鼠有免疫促进作用.
-
太子参须多糖对隐球菌感染小鼠中肺部及脑部感染的影响见图 6.模型组与对照组相比,小鼠肺部及脑部的隐球菌数显著增加(p<0.001),治疗组与模型组相比,治疗后小鼠肺部及脑部隐球菌显著降低,说明太子参须多糖可能是通过增强小鼠的免疫功能而产生了治疗作用.
-
由图 7可知,隐球菌感染模型组小鼠肺部TLR4受体和CREB蛋白量显著降低(p<0.01).与模型组相比,太子参须多糖治疗组的CREB和p-CEBP蛋白表达量显著增强(p<0.01),TLR4受体的表达也得到部分恢复.
3.1. 多糖质量浓度测定结果
3.2. 太子参须多糖对巨噬细胞(RAW264.7)增殖的影响
3.3. 太子参须多糖对RAW264.7巨噬细胞吞噬中性红和隐球菌的影响
3.4. 太子参须多糖治疗隐球菌感染小鼠的作用
3.4.1. 小鼠体质量的变化
3.4.2. 血浆中炎症因子水平的变化
3.4.3. 肺部及脑部隐球菌计数
3.4.4. 太子参须多糖对隐球菌感染模型小鼠肺部中CREB和TLR4的影响
-
新型隐球菌是一低毒性病原体,在自然界分布广泛,大多从呼吸道吸入,形成肺部病源,经血流播散于全身各器官,引起人的亚急性或慢性脑膜炎症[7-8].该病治疗较为困难,死亡率高达20%~30%.由新型隐球菌感染的马、牛、羊、猪、狗、猫的病例已先后在国内外被报道.寻找一类通过增强巨噬细胞的促炎反应、提高机体抗病能力的物质,是预防该病原微生物感染的有效途径.
新型隐球菌侵入机体后,巨噬细胞能参与机体原发性及适应性免疫应答,杀灭隐球菌,另一方面可吞噬隐球菌,作为机体扩散载体造成全身性感染.在隐球菌的致病机制中,细胞免疫起到关键的作用.它可以吞噬和杀灭菌体、形成肉芽肿,限制菌体扩散、提呈抗原、分泌细胞因子,刺激细胞免疫及隔离隐球菌荚膜多糖抗原等作用[9].有研究报道证明,太子参须多糖粗提物能明显改善环磷酰胺所引起的免疫器官(胸腺、脾脏)及心、肺的损伤[10-13],增强免疫功能.事实上,植物多糖的免疫调节作用被屡屡报道:檀新珠等[14]研究表明太子参茎叶多糖能够提高环磷酰胺所致免疫抑制小鼠的免疫功能.本实验采用MTT法检测,发现太子参须多糖能显著上调RAW264.7巨噬细胞的相对增殖率.经太子参须多糖治疗的隐球菌感染小鼠,其体质量恢复到对照水平,而小鼠肺部和脑部的隐球菌数也明显低于模型组.该结果进一步表明,太子参须多糖能改善小鼠的免疫功能.
细胞因子在炎症和抗炎症反应中起着重要的调节作用. IL-10是一种抗炎性因子,发挥下调炎症反应,拮抗炎性介质对机体的损伤作用[15-16].本实验结果显示,太子参须多糖治疗的小鼠,血浆中IL-10质量浓度较模型组显著提高.因此,太子参多糖可能通过增加抗炎因子IL-10的表达,发挥抗炎作用而减少了肺部及脑部隐球菌的感染.此外,太子参须多糖显著降低血浆中促炎因子IL-6的质量浓度. IL-6不仅可以激活中性粒细胞,而且还能延迟吞噬细胞对衰老和丧失功能的中性粒细胞的吞噬,加剧创伤后炎症介质的产生[17].有研究表明IL-6可通过控制促炎症因子的水平,在局部和全身急性炎症反应中起着关键的抗炎作用.在机体受到刺激产生免疫调节的同时,可刺激T细胞产生IL-1β和TNF-α等细胞因子,在免疫应答和组织修复中起着重要作用.治疗组中IL-1β及TNF-α分泌量相对于对照组有所增加,这可能是巨噬细胞的正向免疫调节作用:通过分泌一些细胞因子(如IL-1,IL-6及TNF-α)调节吞噬功能,抑制促炎因子IL-6的分泌,促进抗炎因子IL-10的分泌,并部分增加细胞因子来调节机体免疫,发挥免疫调节作用[18].
Toll样受体(TLR)作为识别病原体相关分子模式的关键天然免疫受体,在宿主防御系统中发挥着重要作用. TLR4是最早被发现的TLR家族蛋白,也是LPS信号转导通路中至关重要的受体[19-20].本研究发现太子参须多糖能增强被隐球菌感染的小鼠肺部的TLR4表达. TLR4活化后,进一步激活cAMP反应元件结合蛋白(CREB),该蛋白作为重要的转录调控因子,促进白介素IL-10和TNF-α的合成.事实上,各种炎症刺激包括LPS、促炎细胞因子、氧自由基、病毒等都能激活CREB[21].而活化后的CREB能进一步促进炎症介质和促炎细胞因子的转录表达.因此,我们初步推测,肺部中TLR4/CREB途径极可能是隐球菌诱导炎症反应的物质基础.这些发现有助于后续关于太子参须多糖治疗相关炎性疾病的机制研究,为其更为广泛的临床应用提供了有力的理论依据.
-
太子参须多糖能增强RAW264.7巨噬细胞的增殖,以及巨噬细胞对中性红和隐球菌的吞噬能力;在隐球菌感染小鼠中,经太子参须多糖治疗后能恢复小鼠的体质量与对照组相近,减少肺部及脑部隐球菌数量,上调肺部中TLR4受体及CREB的表达,并能增加小鼠血浆中IL-10的分泌,减少IL-6的分泌.该多糖有望进一步被开发为一种功能性食品添加剂,用于提高动物机体抵抗病原微生物感染的能力.本研究结果显示出太子参须多糖短期的免疫增强作用,太子参须多糖对隐球菌感染小鼠长期的免疫增强作用仍需进一步研究.










 DownLoad:
DownLoad: