-
慢性变应性接触性皮炎为皮肤科常见病、多发病,是因长期反复接触某一弱刺激性、低浓度致敏物质引起的皮肤炎症.在接触部位呈现红斑、水肿等炎性表现,病理可观察到炎细胞浸润,因此认为炎细胞的募集在变应性接触性皮炎的发病与进展中占主导地位.临床实践中,抗炎是治疗变应性接触性皮炎的有效方法之一.中药四季青在一些研究中被证实具有良好的抗炎作用[1-2],亦被配伍用于接触性皮炎的治疗[3],《中国药典》(2015版)提示无毒性,因此四季青成为治疗慢性变应性接触性皮炎的待试药物.
皮肤是保护体内器官免受外界环境有害物质刺激的重要生理屏障,当受到外界因素刺激时,能分泌多种炎症因子以积极参与局部甚至全身炎症反应.长期反复接触2,4-二硝基氟苯(DNFB)可引起表皮损伤,肉眼可见红斑、水肿等炎性损伤的体征,多种细胞因子异常表达共同影响其发生、发展和转归[4],如白细胞介素18(IL-18)、白细胞介素1β(IL-1β)等.也有研究指出,黑色素瘤缺乏因子2(AIM2)的高表达是慢性接触性皮炎反复发作的机制之一[5]. AIM2/半胱氨酸蛋白酶(Caspase-1)组成炎症小体,分泌炎性因子IL-1β,IL-18.故本课题拟基于四季青的抗炎作用和AIM2炎症小体信号通路的特征,观察四季青水提液对DNFB诱导大鼠慢性变应性接触性皮炎的治疗作用及作用机制.
HTML
-
四季青饮片购自重庆市中药研究院门诊部.称取2 kg四季青饮片,加入纯化水10 000 mL,浸泡30 min后加热至100 ℃回流提取2 h,趁热过滤,收集滤液9 200 mL,残渣加入9 000 mL纯化水,采用同样方法,收集第2次滤液8 700 mL,合并2次滤液共计约17 900 mL,采用旋转蒸发仪浓缩至1 390 mL,得生药约1.44 g/mL(以长梗冬青苷计不低于10.41 mg/g),分装后-20 ℃冷冻保存.
-
DNFB,东京化成工业株式会社生产;丙酮,重庆川东化工有限公司生产;大鼠IL-1β,IL-18 ELISA检测试剂盒,均购于Shanghai Jinjin Chemistry Technology Co.,Ltd;AIM2,Caspase-1一抗及羊抗兔二抗均购于武汉伊莱瑞特生物科技股份有限公司;ST360酶标仪,上海科华实验系统有限公司;Mass2000图象处理系统,四川大学图象处理国家研究所;荧光定量PCR仪,BIOER公司.
-
SD大鼠,SPF级,由重庆市中药研究院实验动物研究所提供,生产许可证号为SCXK(渝)2017-0003.在温度为20~26 ℃、相对湿度为40%~70%的饲养环境下饲养,使用许可证号为SYXK(渝)2017-0004.实验中对动物的处置符合动物伦理学要求.
1.1. 受试药物
1.2. 试剂与仪器
1.3. 动物
-
SD大鼠(雌雄各半,体质量180~220 g)检疫及适应性饲养后,参照文献[6]脱毛、DNFB致敏,2周后DNFB激发,每周激发1次,共4次,大鼠背部可见明显红斑、水肿、结痂、抓痕、鳞屑,即得到慢性变应性接触性皮炎模型.另8只大鼠(正常大鼠)不作处理.
-
选取造模成功大鼠32只,随机区组法分为4组(每组8只),即模型对照组,四季青水提液高、中、低剂量组(每公斤含生药14.4 g,7.2 g,3.6 g);取正常大鼠8只设为正常对照组.各组灌胃给予相应受试药物(正常对照组、模型对照组给予等体积纯化水),给药体积10 mL/kg,每日1次,给药周期14 d.
-
各组动物于首次给药前、治疗7 d后、末次给药后24 h(观察终点),肉眼观察皮损情况,采用EASI评分方法[7]评分,考察症状包括红斑、水肿、结痂,以症状总积分来判断组间差异,尼莫地平法[8]计算各组总有效率;造模前、首次给药前、治疗第7 d后、末次给药后24 h,颞丛静脉采集血液0.5 mL,3 000 r/min离心,取上清,ELISA检测IL-18,IL-1β水平;安乐处死大鼠后,迅速剪取造模局部皮肤,取0.2 g实时荧光定量PCR检测皮损中AIM2,Caspase-1相对表达量,剩余造模局部皮肤组织以中性甲醛固定,HE染色,定性观察组织病理改变,并对炎细胞数进行定量分析.
AIM2,Caspase-1相对表达量检测方法:使用Trizol总RNA提取试剂盒获得皮损组织中的总RNA,逆转录为cDNA. AIM2引物序列:上游5'-AAAGCCCAAAACTAAGGTG-3',下游5'-TCTGCCATACTTATACCCTC-3';Caspase-1引物序列:上游5'-TAAATGGATTGCTGGATGAAC-3',下游5'-TCGTGCCTTTTCCATAACAGT-3';β-actin引物序列:上游5'-CCCATCTATGAGGGTYACGC-3',下游5'-TTAATGTCACGCACGATITC-3'.采用DBI三步法PCR扩增标准程序扩增40个循环,行荧光定量PCR,根据公式2-ΔΔCt计算目的基因的相对表达量.
-
IL-18,IL-1β,AIM2,Caspase-1,炎细胞数以x±s表示,总有效率以%表示;IL-18,IL-1β,AIM2,Caspase-1,炎细胞数以t检验进行数据统计,总有效率以Ridit分析进行数据统计;统计软件SPSS 19.0.
2.1. 制备模型
2.2. 分组及药物干预
2.3. 指标观察及测定
2.4. 统计学处理
-
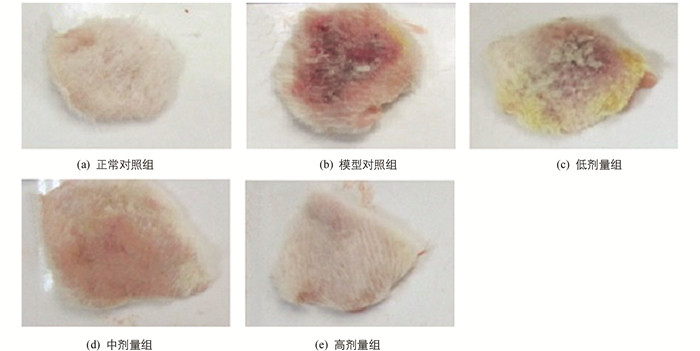
皮损症状积分结果及皮损疗效总有效率见表 1,观察终点皮损局部情况见图 1.模型对照组治疗7 d后、末次给药后24 h与首次给药前比较,差异无统计学意义.与模型对照组比较,在治疗7 d后高剂量组皮损积分明显降低,末次给药后24 h中、高剂量组皮损积分均明显降低;至观察终点,与模型对照组比较,中、高剂量组总有效率差异有统计学意义.
-
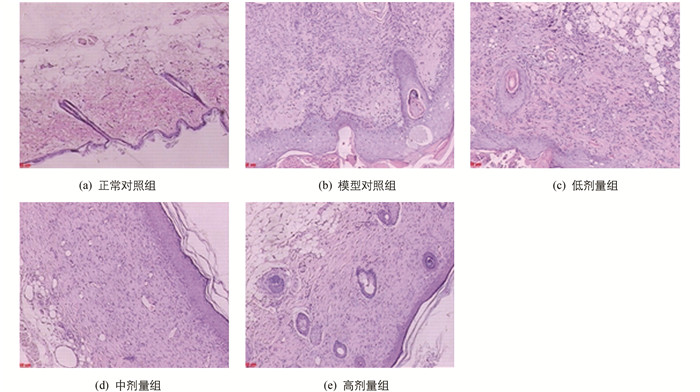
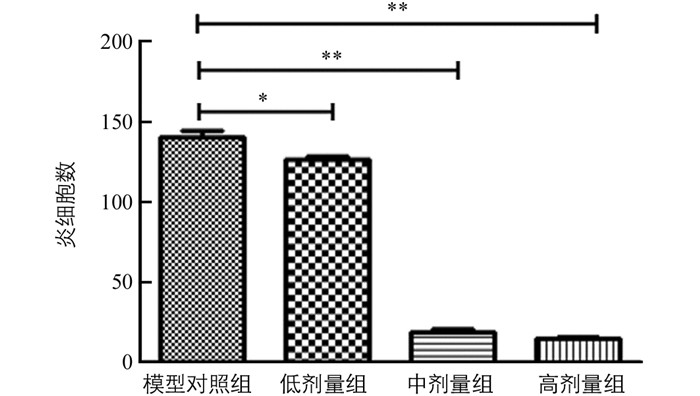
结果见图 2及图 3.定性观察:正常对照组动物皮肤组织结构正常,未见明显病理变化;模型对照组、低剂量组动物造模局部表皮真皮纤维组织增生和肉芽组织形成,炎性细胞浸润累及皮下组织;中、高剂量组动物造模局部表皮真皮纤维组织增生修复良好.定量分析:与模型对照组比较,低、中、高剂量组炎细胞数减少,差异有统计学意义.
-
结果见表 2和表 3.造模前,模型对照组、低剂量组、中剂量组、高剂量组与正常对照组比较,各组血清IL-18,IL-1β水平差异均无统计学意义.首次给药前,模型对照组、低剂量组、中剂量组、高剂量组与正常对照组比较,各组别血清IL-18,IL-1β水平均升高,差异有统计学意义.治疗7 d后,低剂量组、中剂量组、高剂量组与模型对照组比较,高剂量组血清IL-18,IL-1β水平均下调,差异有统计学意义.末次给药后24 h,低剂量组、中剂量组、高剂量组与模型对照组比较,中、高剂量组血清IL-18水平下调,差异有统计学意义;高剂量组血清IL-1β水平下调,差异有统计学意义.
-
结果见表 4.模型对照组与正常对照组比较,模型对照组AIM2,Caspase-1均过表达,差异有统计学意义.与模型对照组比较,通过四季青水提液干预后,高剂量组AIM2表达下调,差异有统计学意义.
3.1. 皮损评分结果
3.2. 组织病理学检查结果
3.3. 对血清IL-18,IL-1β水平的影响
3.4. 对大鼠皮损AIM2,Caspase-1相对表达量的影响
-
减少或避免致敏物质的接触是根治慢性变应性接触性皮炎的有效方法,这一方法操作起来并不现实,因一些过敏物质逐渐被发现而“隐性”存在于多种物品中,如纹身可致慢性变应性接触性皮炎(色素降解产物致敏)[9]、牙医接触牙科材料致接触性皮炎(树脂改性玻璃离聚物致敏)[10],因此药物的筛选仍有意义.模型SD大鼠灌胃给予待筛药物四季青水提液,在治疗结束后可见中、高剂量组皮损积分较模型对照组显著降低、总有效率较模型对照组显著增加、炎细胞数较模型对照组明显减少,表明四季青水提液对DNFB诱导慢性变应性接触性皮炎大鼠炎性皮损有良好的改善作用.
炎症小体是炎症研究中的一个里程碑式的发现,其家族成员众多,AIM2是其中的一员. AIM2可存在于细胞质和细胞核内,其N端含有热蛋白结构域(PYD),在C端为核苷酸/寡糖结合结构域,是HIN200家族的成员[11]. AIM2能够特异性地识别细胞质当中变异或异位的DNA分子,包括受损的DNA分子以及由于细胞核核破裂而泄露在胞浆当中的DNA片段[12].皮肤中的AIM2通过其PYD结构识别结合细胞浆DNA而激活,释放炎症因子.基因敲除和基因沉默AIM2后,发现促炎细胞因子的产生明显减少,这表明AIM2对于炎症因子的产生或释放发挥重要作用[13].在接触性皮炎患者皮损中可检测出AIM2表达的升高[14],提示AIM2炎症小体在慢性变应性接触性皮炎中发挥了促炎作用.本研究模型对照组AIM2表达较正常对照组明显升高,差异有统计学意义,模拟了慢性变应性接触性皮炎AIM2异常的疾病过程.通过四季青水提液的干预,高剂量组较模型对照组AIM2表达显著降低,表明四季青水提液对慢性变应性接触性皮炎大鼠AIM2有抑制作用.
Caspase-1属Caspase家族成员,是炎症小体活化的必备“元件”. Caspase-1既被认为是IL-1β的转化酶,也被认为其对IL-18的成熟分泌起关键作用,因此炎症小体活化的最终结果是IL-18和IL-1β的剪切成熟[15]. IL-18,IL-1β同属IL-1家族,IL-1细胞因子被认为是协调急性和慢性炎性疾病的第一个细胞因子家族[16]. IL-18参与Th1细胞活化,但也涉及产生Th2的γδT细胞和巨噬细胞活化[17]. IL-1β促进白细胞的浸润、促进T细胞和B细胞的增殖分化等[17].炎症因子在炎症反应中起着重要作用[18],IL-18,IL-1β共同促进了炎症反应,抑制这些炎性因子可减缓炎症损伤.经四季青水提液干预后,高剂量组较模型对照组IL-18,IL-1β水平明显下调,表明四季青水提液对慢性变应性接触性皮炎大鼠IL-18,IL-1β过表达有抑制作用.
综上,本研究表明,四季青水提液对慢性变应性接触性皮炎大鼠有治疗作用,其机制可能是通过抑制AIM2/Caspase-1炎症小体信号通路来实现的.






 DownLoad:
DownLoad: