-
微孢子虫作为一类专性细胞内寄生、密度依赖性的单细胞真核寄生虫,可寄生几乎所有的动物(包括人类)和部分原虫[1-2]. 2009年,Tourtip等[3]从生长缓慢的斑节对虾中分离、鉴定了一种新的微孢子虫并命名为虾肝肠胞虫Enterocytozoon hepatopenaei. 该微孢子虫随后在各对虾养殖国家,特别是亚洲地区进行快速传播,已成为影响凡纳滨对虾和斑节对虾育种、育苗及养殖的重要病原.
微孢子虫的孢外感染期为休眠孢子,具有由几丁质和蛋白等组成的厚厚的孢壁,一般的药物难以透过造成杀伤. 微孢子虫侵染时通过发芽的方式弹出侵染装置——极管,将具有侵染性的孢原质输入到宿主细胞内,或孢子利用內吞途径进入宿主细胞进行增殖[1]. 微孢子虫的胞内增殖期分为裂殖增殖期和孢子形成期,具包膜或直接与宿主细胞质接触,是微孢子虫快速增殖的时期. 鉴于微孢子虫独特的细胞结构和生活史,至今尚未开发出特异有效的治疗药物. 应对微孢子虫病最有效的措施在于防控,而检测是诊断和控制疾病流行的重要手段.
各国学者针对虾肝肠胞虫的检测开展了诸多组织病理学和分子生物学的研究,尤以基于核酸的分子生物学检测法研究较多. 2009年,Tourtip等[3]采用SSU-PCR法鉴定了虾肝肠胞虫. 随后10年间,研究者们报道了虾肝肠胞虫的原位杂交法、基于核糖体小亚基SSU rDNA,孢壁蛋白SWP,微管蛋白β-tubulin基因序列的巢式PCR法[4-7]、SYBR Green Ⅰ实时荧光定量PCR法[8]、TaqMan实时荧光探针定量检测法[9-11]、环介导等温扩增法(Loop-mediated isothermal amplification,LAMP)及其衍生的多种实时或可视化产品[12-16]、重组酶介导的核酸快速扩增法(Recombinase-aid amplification,RAA)[17]等. 在组织病理学检测方面,常晓晴等[18]通过比较8种不同染色方法提出Masson(马松)染色法最适于检测病理组织切片中的EHP.
在实际应用时,除了考虑检测方法的特异性、灵敏性和简便性,检测成本和效率因素也常被纳入评估标准. 宋增磊等[19]推荐在定性上采用50∶1的并样进行qPCR检测以缩短检测时间和降低成本. Cruz-Flores等[20]采用1 mL注射器在对虾肝胰腺的特定位置针刺取样,实现对虾的非致命抽样,可用于亲虾的检测,评估其无特定病原体(Specific pathogen Free,SPF)状态. Thamizhvanan等[21]采用多重PCR技术可同时检测样品中的EHP和对虾白斑综合征病毒(White spot syndrome virus,WSSV),提高了多病原检测的效率,降低了检测成本. Karthikeyan等[22]报道了采用FTA卡(WhatmanTM FTA cards)在常温下进行样品核酸收集、运输和纯化以替代送检过程的低温活体运输,降低了送检成本.
以上方法的建立,无疑对于虾肝肠胞虫的检测及防控具有重要意义,并且已应用于对虾养殖各个环节的跟踪检测. 特别是实时荧光定量PCR技术是目前病原分子检测的金标准. 2020年,Zhao等[23]报道了利用Calcofluor white检测对虾粪便和组织样品中的EHP孢子,此方法将推动EHP的快速镜检. 本研究主要基于微孢子虫孢子在光镜下具有高度折光性、孢壁结构的特殊性以及孢原质膜的特殊透过性,通过比较普通光镜成像、微分干涉差成像以及单、双色荧光显微成像的光镜检测方法,为虾肝肠胞虫的早期筛查及养殖塘样品的快速诊断提供价廉、省时且易操作的检测手段.
HTML
-
生长滞后的凡纳滨对虾取自广东省沙堆镇及大鳌镇养殖塘. 采集时取样品肝胰腺均分后液氮速冻,干冰运输至微孢子虫感染与防控重庆市重点实验室,-80 ℃保存备用.
-
取检测EHP阳性的肝胰腺组织备样置于干净的1.5 mL离心管中,采用手持式微量匀浆器(LABGIC,TH-Mini)处理30 s,获得样品悬液待用.
-
匀浆处理后的待检样品悬液制备为临时装片,采用微分干涉差显微镜检查法进行检测. 取移液器吸取10 μL或蘸取少量样品悬液,用水或PBS溶液稀释10倍后滴加于干净载玻片,盖上盖玻片制成临时装片,采用带有100倍微分干涉差(Differential interference contrast,DIC)镜头的光学显微镜(OLYPUS,BX53F)进行普通光学观察和微分干涉差观察.
-
匀浆处理后的待检样品悬液,采用荧光增白剂染色法或双色荧光法进行检测. 荧光增白剂Fluorescent Brightener 28(sigma)母液(1 mg/mL)用水稀释1 000倍为几丁质染色液备用. 取储存质量浓度为1 mg/mL的碘化丙啶(Propidium Iodide,PI)用几丁质染色液稀释1 000倍为双染工作液(现配现用). 取样品与50 μL染色液混匀,室温静置孵育5 min,吸取适量滴加于多聚赖氨酸包被的载玻片制成临时装片,置荧光显微镜下(OLYPUS,BX53F),分别选取紫外光和绿色光激发滤板进行观察.
1.1. 实验材料
1.2. 样品处理
1.3. 普通光镜样品制片及观察
1.4. 荧光光镜样品处理及观察
-
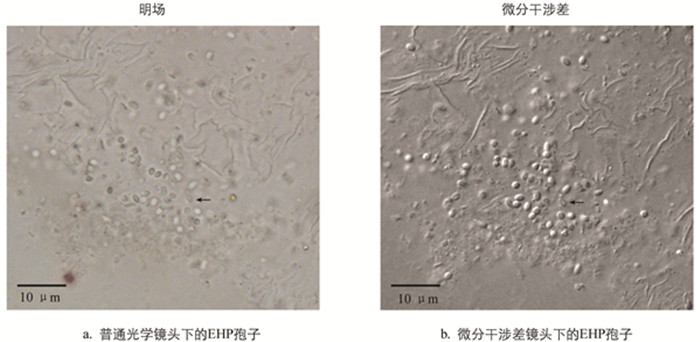
在光学显微镜下,虾肝肠胞虫感染的肝胰腺组织中可看到孢子存在,呈浅绿色折光性(图 1). 相较于普通的光学显微观察而言,微分干涉差(DIC)显微观察下孢子的形态更清晰和立体,与组织间的界限更明显,且可分辨出发芽后的孢子空壳,如图 1中黑色箭头所示. 发芽后的孢子因孢原质弹出而不再饱满,折光性丧失,中心区域与边缘呈现出明显的界限. 发芽是微孢子虫特有的侵染方式,所以可以通过发芽与否将微孢子虫孢子与其他真菌孢子区别开来. DIC下观察到的孢子空壳可作为虾肝肠胞虫的佐证.
-
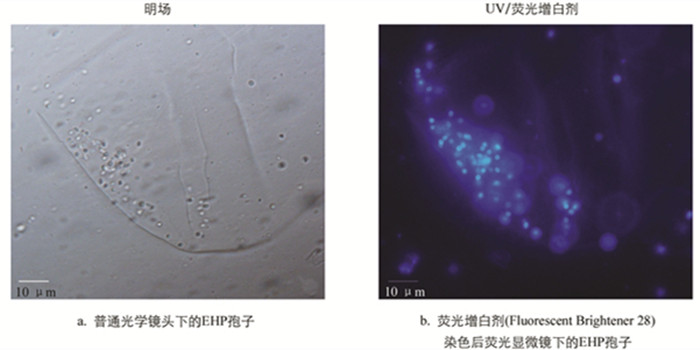
微孢子虫孢内壁为增厚的几丁质层,可与荧光增白剂(Fluorescent Brightener 28,FB 28)结合,在紫外激发光下呈现出明显的蓝色荧光,这也是其区别于真菌孢子的重要特征之一. 如图 2所示,经FB 28染色后的肝胰腺组织匀浆,在紫外激发光下可见清晰的卵圆形孢子,孢壁均匀着色与背景形成强烈对比.
-
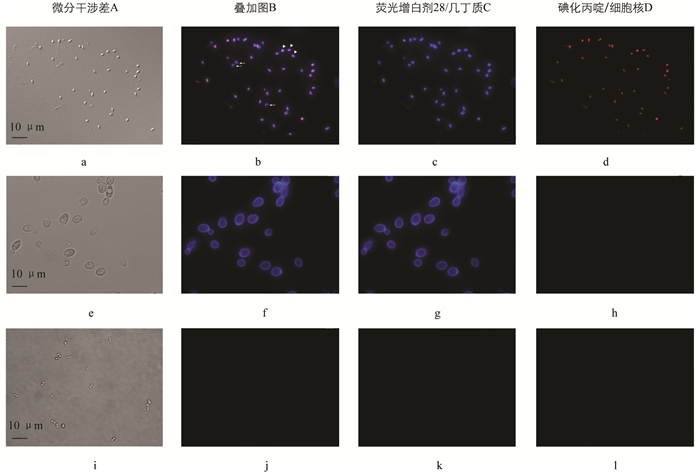
采用FB 28和PI对虾肝肠胞虫样品进行染色后,可见同一孢子内几丁质层标记为蓝色荧光,细胞核标记为红色荧光(图 3). 发芽后的孢子由于孢原质已输出,仅留下孢壁结构,因此可见仅染上蓝色荧光的孢壳(图 3). 由于FB 28和PI处于游离状态时荧光较弱,分别与几丁质和DNA结合后才可见强烈的聚集性荧光,且微孢子虫孢子细胞膜的特殊透过性使PI能够进入活孢子内部进行染色,因此实验过程不需要进行样品的固定和洗涤.
以真菌酿酒酵母Saccharomyces cerevisiae和分离自对虾肠道的细菌乙酰微小杆菌Exiguobacterium acetylicum进行活细胞染色开展对比实验. 如图 3可见,酿酒酵母因其细胞壁中含有几丁质成分,能够被FB 28染上蓝色荧光,但是PI不能透过酵母细胞壁,且酵母细胞与虾肝肠胞虫孢子细胞大小、形态具有明显的区别,显微镜下荧光强度亦弱于虾肝肠胞虫孢子. 乙酰微小杆菌因不含有几丁质成分则不能被FB 28染色,PI亦不能透过细菌细胞壁对细胞核进行染色.
2.1. 微分干涉差显微成像
2.2. EHP的单色荧光显微成像
2.3. EHP的双色荧光显微成像
-
虾肝肠胞虫Enterocytozoon hepatopenaei,EHP感染不会引起对虾如凡纳滨对虾、斑节对虾等快速死亡. 对虾感染EHP后主要表现出生长缓慢或停滞的症状,故而延长养殖周期,增加养殖户的经济投入,同时对虾发育不齐导致经济效益大打折扣,给养殖户造成巨大的经济损失. 对虾育种、育苗过程中,EHP可从亲虾传染到子代且很难从培育体系中去除[24]. 虾苗携带EHP,特别是在高密度养殖情况下影响对虾发育,且EHP感染易伴随其他病原体如传染性皮下及造血组织坏死病毒(Infectious hypodermal and hematopoietic necrosis virus,IHHNV)[25]、白斑综合征病毒(White Spot Syndrome Virus,WSSV)[21]和弧菌[26]等的合并感染,从而进一步扩大危害.
目前没有可杀灭微孢子虫又不影响宿主生长的特效药物,仅有烟曲霉素及其类似物、苯并咪唑(如阿苯达唑)、托曲珠利、硝唑尼特、莫能菌素等对部分物种有一定的抑制效果[1]. 因此,养殖前的消毒防控,养殖中的环境及饲料保障、跟踪检测并及时废弃仍然是目前防控微孢子虫病最有效的方法. 以往的研究者采用特异性抗体通过免疫荧光或免疫印迹等方法实现了微孢子虫的特异检测[27-28]. 随后针对孢子表面特异蛋白和核酸等开发出了一系列特异灵敏的分子检测方法,这些方法也在对虾养殖业中应用到了EHP检测,尤其是实时荧光定量PCR已经成为EHP分子检测的金标准. 这些检测技术主要应用在执行检测职能的事业单位、大型育苗和养殖企业、第三方检测机构,因设备成本和检测人员的专业限制,尚不能应用于养殖一线.
利用光学显微镜对病原进行形态学鉴定是应用最广泛且简便实用的方法. 不同种类的微孢子虫具有大小不一、形状多样的孢子,在光学显微镜下呈现出高度折光性,其形态特征可应用于快速检测. 如应用于蚕业检疫的母蛾镜检法就是利用了家蚕微粒子虫孢子的折光性和已知的大小、形态特点来检测[29]. 微分干涉差或相差显微成像的应用使孢子更易于观察,有助于经过培训的人员进行快速镜检. 但是大多数微孢子虫的孢子很小,且许多寄生虫学中常用的染色方法对微孢子虫孢子着色效果并不理想. 虾肝肠胞虫的孢子为卵圆形,大小为0.7×1.1 μm[3],为镜检增加了难度. 荧光增白剂(Calcofluor white M2R或Fluorescent Brightener 28)可与几丁质的β-1,4糖苷键特异结合,是普遍用于微孢子虫孢子染色的方法[30-31]. 荧光增白剂能使微孢子虫孢子染上强烈的蓝色荧光,细菌和病毒因不具有几丁质而不能着色. 虽然真菌孢子和宿主的几丁质成分也能被其染色,但是荧光较弱或可通过形态进行区分. 采用Fluorescent Brightener 28和Propidium Iodide(PI)双色荧光法对固定后的样品进行染色,可分别对微孢子虫的几丁质层和细胞核进行标记,能进一步排除组织细胞和几丁质碎片的干扰[32-33]. PI一般情况下不能透过细胞膜,主要应用于死细胞的核染色. 本实验室研究发现,微孢子虫膜结构的特殊透过性使得PI能够进入活的微孢子虫对其细胞核进行染色[34]. 因此,本研究所描述的双色荧光标记法摒弃了细胞固定及洗涤的步骤,仅需对组织样品进行简单的匀浆处理和染色孵育,并进一步排除其他具有几丁质层单细胞真核生物的影响. 整个过程简单、快速,总耗时不超过10 min,且操作和结果判读不具有专业性要求,单次检测成本极低,可视化的荧光显微镜亦在国内有量产,非常适合在苗场及养殖一线的服务站中推广. 通过荧光筛查,可初步判断养殖塘出现生长缓慢综合征的对虾样品是否可能由虾肝肠胞虫感染所致,可对病情做出及时判断,降低养殖风险. 阳性结果再辅以核酸定性、定量检测. 采用该方法,即使只有1个孢子也能通过染色观察进行快速镜检,且可对分子检测灵敏度下限的样品进行快速的补正检查. 通过本研究,可为虾肝肠胞虫病的检测提供简便、快速、实用的方法.






 DownLoad:
DownLoad: