-
开放科学(资源服务)标识码(OSID):

-
能源危机和环境污染的日益严重促进了光电催化材料的发展[1],因此,对于高效率催化剂的开发显得尤为重要. 过渡金属硫化合物如硫化钴、硫化镍、硫化钼等[2-3]因其独特的结构特征和光电性质,被广泛应用于光电催化中. 其中,Co9S8由于出色的氧化还原能力、窄的带隙、高的导带位置和有效的电荷转移被认为是催化领域重要的催化材料之一[4-5]. 然而,通常制备的Co9S8由于比表面积小,活性位点暴露不足,因而限制了其进一步应用[6].
气凝胶作为一种具有超低密度、高孔隙率和大比表面积的三维多孔固态材料,自1930年问世以来一直受到研究者的高度关注[7],如SiO2气凝胶[8]、金属氧化物气凝胶[9]、金属气凝胶[10]、石墨烯/碳气凝胶[11-12]等,由于具有质量轻、光折射率高、内阻小、储量大、充放电能力强等优点,在隔热阻燃、能量储能、航天航空等领域都有重要应用. 可以预见,拥有纳米多孔网络结构、巨大的比表面积和介观尺度结构可控的气凝胶应用于催化反应,能够显著增加反应的活性位点,加快物质/电荷的转移和传输,从而大大提升材料的催化效率[13].
本研究采用溶胶—凝胶法结合低温处理制备了多孔疏松的Co9S8气凝胶(CSA),与CSS相比,CSA具有更大的比表面积和孔容量,在OER反应过程中可以提供更多的反应活性位点及良好的离子输运通道,从而提升OER反应速率. 在光催化降解亚甲基蓝(MB)的测试中发现,CSA在可见光下能比CSS产生更大的光电流响应,有效提高了催化效率. 该成果为拓展具有双催化功能的Co9S8气凝胶的进一步应用奠定了理论和技术基础.
HTML
-
所用化学品和试剂均为AR级分析纯,无需纯化. 将5 mmol Co(NO3)2·6H2O与6 mmol巯基丁二酸分别溶解于5 mL乙醇中,并加入0.5 mL甲酰胺,室温搅拌2 h,倒入体积为20 mL的玻璃容器中. 将溶液置于60 ℃的烘箱内形成溶胶,待溶胶形成后加入乙醇,并每隔24 h更换一次新鲜的乙醇,持续7 d以使凝胶老化,再用去离子水多次离心置换乙醇,得到水凝胶. 将水凝胶置于-20 ℃的冰箱内冷冻24 h,然后放入冷冻干燥机内放置36 h,得干燥的气凝胶. 将气凝胶在Ar气下以5 ℃/min升温至350 ℃,保温2 h,冷却至室温得到黑色粉末,标记为CSA. 同理,将在700 ℃煅烧的样品标记为CSA-700,将未经煅烧的气凝胶标记为CSA-0.
Co9S8纳米片(CSS)的制备过程:在70 mL去离子水中加入1 mmol CoCl2·6H2O和1 mmol硫脲,搅拌至完全溶解;将该溶液密封在体积为100 mL的含聚四氟乙烯内胆的高压釜中,在160 ℃烘箱中放置24 h;反应结束后自然冷却至室温,分别用去离子水和乙醇将产物离心清洗数次,然后置于60 ℃烘箱中干燥12 h,得到黑色粉末样品.
-
用X-ray衍射仪(XRD)分析样品物相结构,扫描速率为5°/min,扫描范围2θ角度为10° ~ 80°. 分别采用扫描电子显微镜(SEM)和透射电子显微镜(TEM)观察样品的微观形貌和晶体结构. 用X射线光电子能谱(XPS)采集样品的化学状态. 采用紫外—可见(UV-Vis)分光光度计检测光催化降解效率. 采用Brunauer-Emmett-Teller(BET)方法在氮吸附仪上分析样品的孔径分布和比表面积. 用CHI760E电化学工作站进行电化学和光电化学测量. 在三电极体系中,以石墨棒为对电极,以Ag/AgCl(0.197V)为参比电极,在O2饱和的1.0 M KOH电解质中记录OER反应. 准备工作电极时,将5 mg催化剂粉末分散在1 mL混合溶液中(490 μL水,490 μL乙醇和20 μL 5 wt% Nafion),超声30 min得到均匀的油墨. 将3 μL墨水滴到用氧化铝粉末抛光的3 mm玻碳电极(GC)上. 线性扫描伏安法(LSV)以5 mV/s的扫描速率进行,本文中所测得的电压值都已转换为相对于可逆氢电极(RHE)的电压值,并采用iR补偿校准了极化曲线,以消除溶液电阻的影响. 在0.5mol/L Na2SO4溶液中以0.204 V vs Ag/AgCl偏压每间歇20 s对样品进行Xe灯照射,并记录样品在持续照射20 s下的电流响应信号. 将5 mg样品加入含有50 mL(20 mg/L)MB溶液的烧杯中,并暴露在由300 W Xe灯模拟的太阳光照射下,记录在给定时间间隔内UV-Vis光谱中MB最大吸收带(664 nm)的变化.
1.1. 样品制备
1.2. 表征分析
-
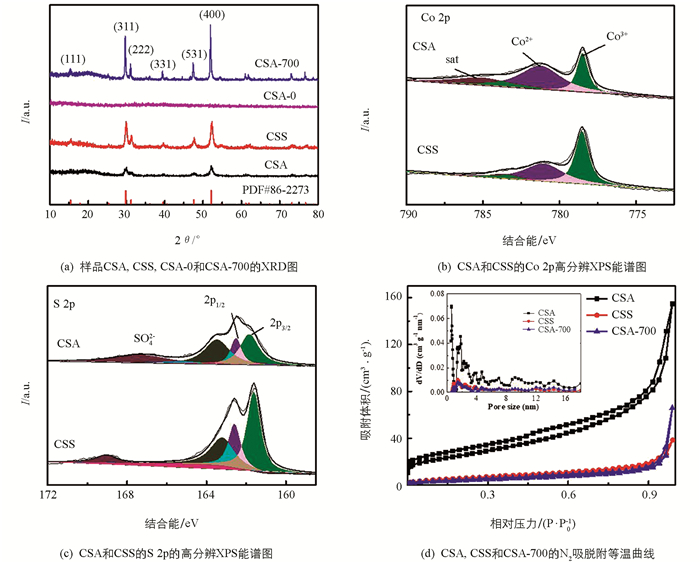
图 1(a)是样品X衍射结果,CSA和CSS的XRD衍射峰与Co9S8(PDF#86-2273)标准卡相对应. 其中位于15.5°,29.8°,31.2°,39.6°,47.6°和52.1°处的衍射峰,分别代表立方结构Co9S8的(111),(311),(222),(331),(511)和(440)晶面. CSA-0没发现明显的衍射峰,表明样品为非晶态,而CSA-700显示出了比CSA更高的衍射峰强度,说明随温度升高,气凝胶的结晶度也随之提高. 与水热法制备的纳米片CSS相比,溶胶—凝胶法制得的气凝胶没有高温高压的生长环境,因此需要进一步晶化处理才能得到相应的结晶材料[14].
图 1(b)为Co 2p的高分辨XPS谱图,结合能在778.5 eV和793.6 eV处的峰应分别对应于Co3+ 2p3/2和2p1/2,在781.3 eV和797.4 eV的两峰也分别为Co2+ 2p3/2和2p1/2,而结合能在785.4 eV与803.3 eV处的两个峰属于强卫星峰[15]. 图 1(c)中S 2p轨道位于161.8 eV(2p3/2)和162.5 eV(2p1/2)的两个拟合峰,通常被认为是属于金属硫化物中的S2-[16],结合能为162.8 eV处的峰与低配位时表面的硫离子有关[17],而位于167.2 eV处的拟合峰对应于样品表面被氧化后得到的硫氧化物.
图 1(d)为样品的BET测试结果,可见CSA(91.2 m2/g)的比表面积是CSS(21.2 m2/g)的4倍多,并且CSA的孔容(0.19 cm3/g)也远大于CSS(0.044 cm3/g),表明气凝胶具有大的比表面积和发达的孔结构. 与CSA相比,CSA-700的比表面积(15.7 cm2/g) 和孔容(0.046 cm3/g)大幅减小,孔径分布也发生明显变化,部分尺寸在1 nm左右的微孔急剧减少或消失,只留下少量更大尺度的微孔,表明CSA在高温下容易团聚结块,导致孔隙结构坍缩,比表面积减小. 以上结果表明,在350 ℃低温处理的样品具有更丰富的多孔网状结构和稳定的结晶相.
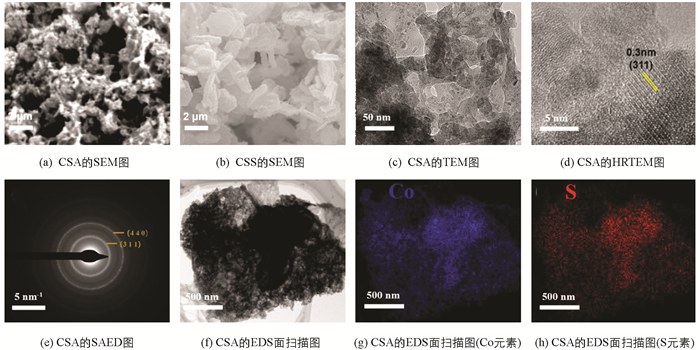
从图 2(a)CSA的SEM图可以看出,样品具有由大量纳米颗粒组成的凝胶骨架结构,其中含有丰富的孔隙. 图 2(b)是水热法制备的Co9S8的SEM图,样品呈纳米片状,表面规则、尺寸均匀,虽也有较大的比表面积,但不具有孔结构. 图 2(c)样品的TEM结果也证实,CSA是由纳米颗粒堆叠而成的三维网状结构,内含大量孔隙;从图 2(d)~(h)HRTEM、SAED和EDS的结构微观分析,可清楚地观察到样品由Co与S两种元素组成,组分均匀,以及属于Co9S8的(311)晶面的晶格条纹[18],进一步证实溶胶—凝胶法合成的样品经低温处理后得到Co9S8气凝胶.
-
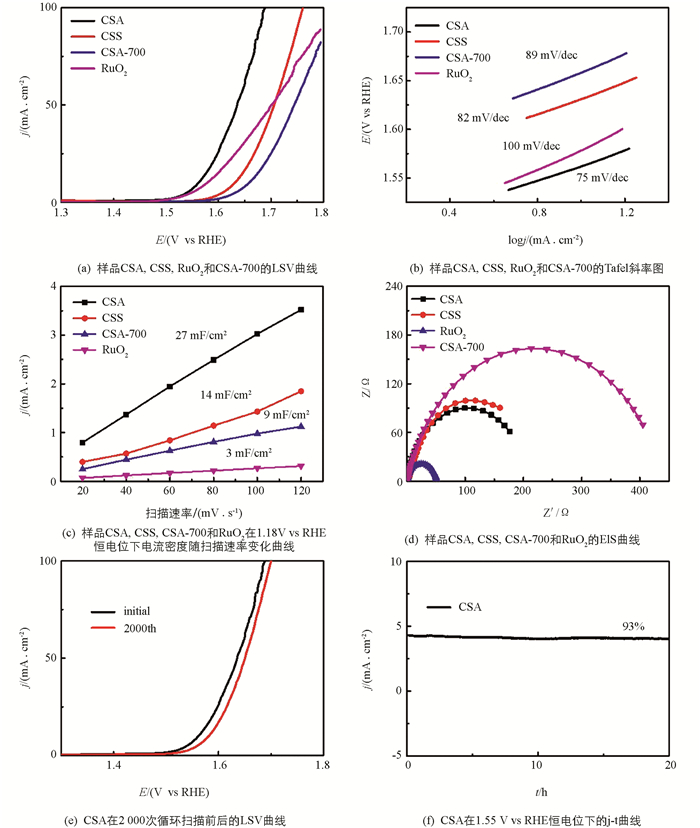
以商业化的RuO2为参考,对所制样品进行OER测试,通过LSV记录了样品的极化曲线,如图 3(a)所示. CSA在达到10 mA/cm2时的过电位为331 mV,这大大低于CSS(400 mV)和RuO2 (347 mV)及CSA-700(427 mV). 图 3(b)进一步分析了CSA的本征催化活性,CSA的Tafel斜率为75 mV/dec,也低于CSS(82 mV/dec)和RuO2(100 mV/dec)及CSA-700(89 mV/dec),说明它具有更优的OER反应动力学性能. 图 3(c)的结果表明,CSA拥有更大的双电层电容(27 mF/cm2),明显大于CSS(14 mF/cm2)和RuO2(3 mF/cm2)及CSA-700(9 mF/cm2),这也是CSA具有优异的OER性能的重要原因之一. 电化学阻抗分析表明,虽然图 3(d)RuO2的Nyquist曲线圆弧半径小于CSA和CSS,但CSA的圆弧半径仍小于CSS和CSA-700,说明CSA具有更好的导电性. 值得一提的是,CSA-700的OER性能对比CSA表现出明显的下降,这是因为经低温调控的CSA保留了气凝胶的典型结构和Co9S8纳米颗粒特有性能,带来了更优异的OER催化活性[19]. 图 3(e)显示CSA在2000次CV扫描后过电位只增加了12 mV,在1.55V vs RHE的长期计时电流法测试后,CSA在20 h内仅有7%的负电流衰减,表明CSA具有良好的OER催化稳定性,如图 3(f)所示.
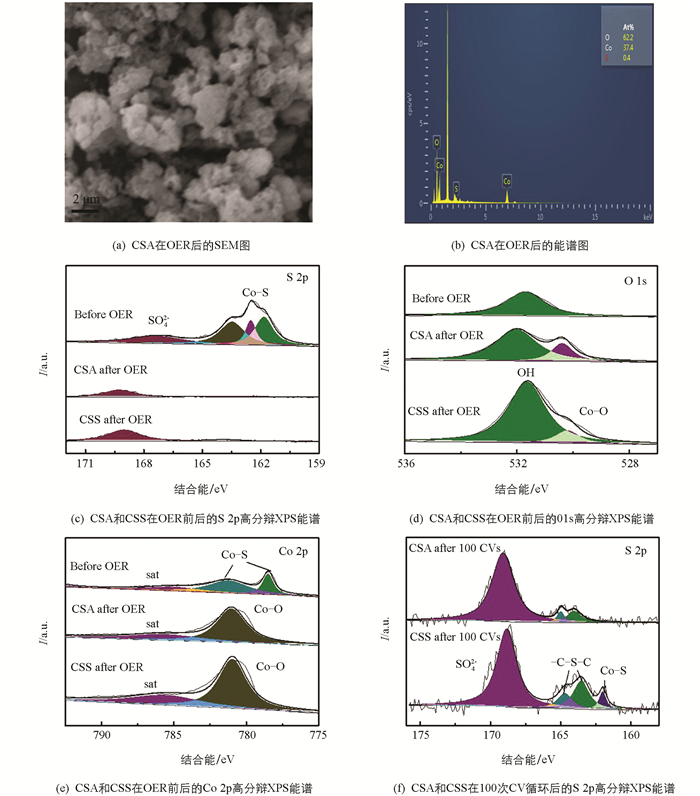
对OER反应后的CSA样品进行SEM检测,以探究催化活性来源,结果如图 4(a)所示. OER反应后样品的形貌和结构没发生明显变化,仍保持着多孔结构特征,但图 4(b)能谱图显示,催化剂中的S元素含量急剧减少. 图 4(c)比较了OER反应前后CSA和CSS的XPS光电子能谱,发现反应前后Co 2p高分辨率XPS谱发生了较大变化,778.5 eV和781.3 eV的Co-S峰在反应后消失,出现了781 eV的Co-O峰. 比较图 4(d)~(e)OER反应前后的S 2p和O 1s的XPS谱图,也发现CSA中属于S2-的峰在反应后全部消失,而在O含量增加的同时,也观察到了Co-O特征峰.
综合以上结果并结合文献报道,可以说明在OER过程中,Co9S8中的S原子与O原子结合,进而不断地通过电化学反应浸出为SO42-并溶解于水中,使得OH-接近Co缺陷位点并最终发生物相变化,形成Co(OH)2/CoOOH作为反应的活性物质[20],该物质已被证明是一种良好的OER催化剂[21]. 测试CSA和CSS进行100次CV循环测试后的S 2p的XPS能谱,如图 4(f),发现CSA中属于Co-S的峰已消失,仅存-C-S-C-[164 eV (2p3/2),165 eV(2p1/2)]和被氧化的SO42-[22],而CSS中仍含有少量的Co-S峰,这表明CSA在OER过程中能更快地进行表面重构形成反应活性中心. 以上结果表明,CSA特有的大比表面积与丰富的孔结构暴露了更多的阴离子(S2-),可加速其刻蚀和溶解以产生更多空位,能加快电子/物质的转移以及反应物表面的自我重构,促进OER反应过程[23]. 此外,有研究者证明,催化剂表面重构后,溶解在电解液中的SO42-还可以提升OER反应速率[24].
-
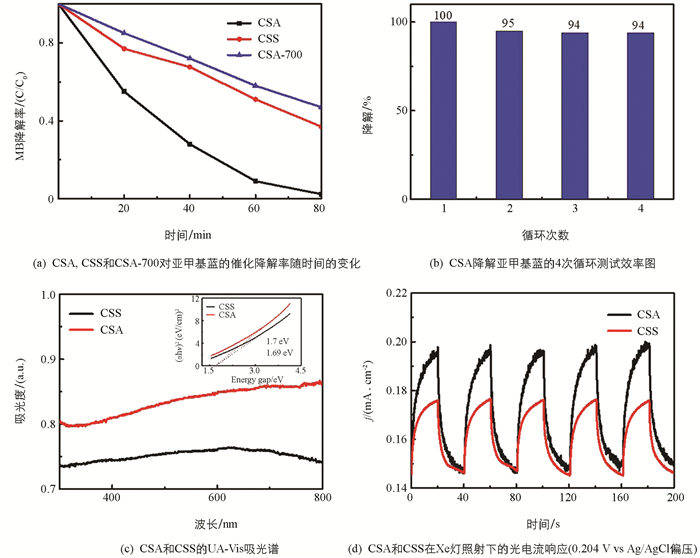
采用UV-Vis紫外可见分光光度计,以亚甲基蓝在664 nm的吸收峰变化来研究制备样品对降解MB的效率. 如图 5(a)~(b)所示,80 min后CSA,CSS,CSA-700这3种样品分别使MB降解了98%,63%,53%,并在4次循环后CSA仍有94%以上的催化降解效率,表明CSA拥有更优的光催化降解MB的能力和光催化稳定性. 紫外/可见漫发射光谱(DRS)被用于研究样品的光吸收特性以及他们的带隙. 如图 5(c)所示,CSA和CSS在300~800 nm处都显示出宽的吸收边缘,说明Co9S8在UV和可见光区域均具有很强的光吸收能力. CSA与SCS的带隙值是由Tauc曲线α(hν)=A(hν-Eg)n/2估算,计算结果显示CSA的带隙值(1.69 eV)与CSS(1.7 eV)没有明显差异,这表明形貌结构的改变并没有改变Co9S8本征的光学特性[25].
为研究CSA的光催化活性来源,测试了样品的光电流响应,图 5(d)可以观察到两种电极产生的光电流具有可重复的开/关循环响应,并且在光照条件下CSA产生了相较于CSS更大的光电流,而这种结果都来源于CSA更大的比表面积暴露了更多的活性位点,增加了对光的利用率,从而产生了更多的光生电子和空穴. 同时,其丰富的孔结构增加了反应物的扩散效率,使得反应物与反应产物能够快速地在样品之间吸附与脱附,增强了光催化效率.
2.1. 样品的形貌和结构特征
2.2. OER性能分析
2.3. 光催化分析
-
本论文通过溶胶—凝胶法与低温热处理成功制备了具有多孔气凝胶结构的Co9S8,并在OER反应和光降解MB中展现出优异的催化性能. 研究表明,其优异的性能主要缘于CSA大的比表面积和丰富的孔隙结构,这不仅大大地增加了反应物与催化剂之间的接触面积,也暴露了更多的S2-,加速了其电化学反应过程中不断地溶解析出以及样品的表面重构,产生更多的催化活性位点,而且其丰富的孔隙结构还能够加快电荷/物质的转移,从而展现出优异的OER性能. 同时,CSA独特的结构优势有利于产生更多的光电子和空穴,提升光催化降解MB的效率.






 DownLoad:
DownLoad: