-
开放科学(资源服务)标志码(OSID):

-
当前,糖尿病作为四大非传染性疾病之一,已成为一个日益严峻的全球性公共卫生问题. 糖尿病患者需要经常检查血液中的葡萄糖含量,以避免高血糖症导致的多种致命急症,因此,准确、快速地分析血液中葡萄糖的含量极其重要[1]. 电化学传感器因具有快速准确的响应、低制造成本和易于使用等优势,在检测葡萄糖的含量方面成为研究热点[2]. 根据葡萄糖检测原理,电化学传感器分为酶传感器和无酶传感器[3],其中无酶传感器因具有灵敏度高、选择性强、检测限低、响应时间快和可以避免复杂的酶法固定技术等优点而受到极大关注[4].
电极材料是电化学传感器非常关键的组成部分,直接影响电化学催化性能[5]. 迄今为止,人们对贵金属、过渡金属、金属氧化物和氢氧化物等材料制备的电化学传感器进行了大量的研究[6-9]. 例如,NiCo2O4[10],CuCo2O4[11]和MnCo2O4[12]等过渡金属和尖晶石氧化物,由于金属间的协同作用,大量的结构缺陷等特点能够增强电极材料的电导率和稳定性,从而展现出比简单氧化物更为优越的电化学性能. 其中钴锰尖晶石氧化物由于成本低、环境友好和锰的多价态等优点,成为催化材料、电极材料和电化学传感装置等研究领域应用的热点[13-14]. 除材料固有的电化学活性之外,材料的结构和形貌也会影响催化性能[15-18]. 例如,Ma等[19]通过溶剂热法得到多孔MnCo2O4微球用于非水系Li-O2电池. Pettong等[20]通过水热法制备MnCo2O4纳米纤维用于不对称超级电容器. 电沉积是一种操作简便和价格低廉的制备纳米材料薄膜的方法,通过电沉积可以将不同形貌的纳米结构材料均匀地附着在基底表层. Nguyen等[21]研究了通过电沉积在不锈钢上制备的MnCo2O4纳米片的结构演变和电化学响应;Sahoo等[22]利用电沉积制备了MnCo2O4纳米片用于超级电容器. Li等[23]通过电沉积法和煅烧直接制备了负载在碳布上的MnCo2O4纳米片的无粘结剂空气电极.
然而,利用电沉积方法制备负载MnCo2O4纳米片的微纳阵列用于葡萄糖传感器的报道比较少见. 本文以镀金玻璃片为衬底,单分散胶体球为模板,利用硫脲作为还原催化剂[24-25],结合两步电沉积法制备了具有高比表面积的MnCo2O4/Ni分级微纳阵列,并对其形貌特征和电化学性能进行了详细研究. 与MnCo2O4/Ni平面电极相比,MnCo2O4/Ni分级微纳阵列在葡萄糖的电化学测试中表现出优异的电催化性能和稳定性,使其在无酶传感器的应用方面具有较大潜力.
HTML
-
直径为500 nm的单分散聚苯乙烯微球(PSS微球)由Alfar Aesar公司购入;氨水(NH3·H2O)、硫脲(CS(NH2)2)、六水硝酸钴(Co(NO3)2·6H2O)、二氯甲烷、六水硝酸镍(Ni(NO3)2·6H2O)、四水合氯化锰(MnCl2·4H2O)和氢氧化钠(NaOH)等购于重庆钛新化工公司;抗坏血酸(AA)、多巴胺(DA)、尿酸(UA)、草酸(OA)和葡萄糖(glucose)购于美国Sigma-Aldrich化学试剂公司. 实验中全程使用去离子水,所有试剂都是分析级.
ETD-7000离子溅射仪(北京意力博通技术发展有限公司)、202-OOA型电热恒温干燥箱(天津市通利信达仪器厂)、扫描电子显微镜(SEM,JEOL JSM 7100F)、透射电子显微镜(TEM,JEM2010)、能量色散能谱仪(EDS)和X射线光谱仪(XPS,Thermo scientific ESCALAB 250). 电化学试验在电化学工作站CHI660E(上海辰华仪器有限公司)完成.
-
首先,将规格为0.5 cm×2.5 cm×0.05 cm的玻璃片用无水乙醇和去离子水超声清洗并干燥,将干燥后的玻璃片在电流为6 mA的离子溅射镀金仪中溅射10 min. 再将载玻片衬底依次用酒精、去离子水超声清洗1 h,放入硫酸、双氧水的混合溶液(体积比为1∶3)中浸泡10 h后捞出,置于体积比为1∶1∶3的氨水、双氧水、去离子水的混合溶液中超声清洗1 h后放入去离子水中,采用气液界面自组装法[26]在载玻片上组装PSS模板并转移到镀金玻璃片上,最后将带有PSS模板的玻璃片置于干燥箱中干燥.
-
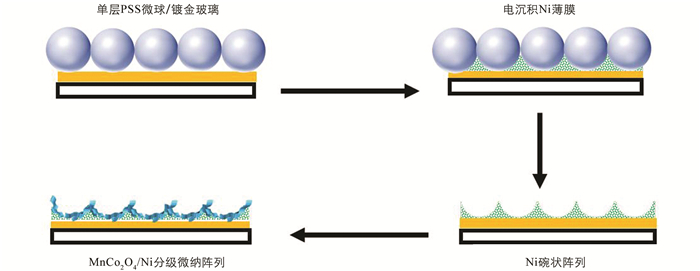
制备MnCo2O4/Ni纳米片复合多孔阵列电极的过程如图 1所示. 首先采用恒电流的电化学沉积方式制备Ni碗状阵列. 以石墨为阳极,制备的胶体球模板为阴极,以0.1 mol/L的NiCl2·6H2O和0.3 mol/L的(NH4)2SO4为电解液,沉积电流为0.8 mA,沉积时间是7 min. 根据有机溶剂易溶于二氯甲烷的原理,将样品在二氯甲烷溶液中浸泡5 min去除胶体球模板,再用去离子水清洗并干燥3 h,最终得到Ni碗状阵列. 最后以Ni碗状阵列为工作电极,银/氯化银(Ag/AgCl)电极为参比电极,铂丝为辅助电极,以15 mmol/L MnCl2·4 H2O,2.5 mmol/L Co(NO3)2·6H2O和0.75 mol/L CS(NH2)2的混合溶液为电解液,采用循环伏安法在Ni碗状有序阵列表面沉积出MnCo2O4纳米片. 电沉积的参数是:电压为-1.2~0.2 V,扫描速率为30 mV/s,实验温度为室温,沉积结束后用去离子水清洗并干燥1 h,由此制得自支撑MnCo2O4/Ni纳米片复合多孔阵列电极. 此外,为了对比分析,用相同浓度溶液和电沉积参数在镀金玻璃片上制备了MnCo2O4/Ni纳米片复合平面阵列.
-
利用三电极系统进行电化学测试,以MnCo2O4/Ni纳米片复合多孔阵列为工作电极,铂丝和银/氯化银(Ag/AgCl)分别为辅助电极和参比电极,采用0.2 mol/L的氢氧化钠溶液作为电解液,对MnCo2O4/Ni电极进行电化学性能评估.
1.1. 试剂与仪器
1.2. 实验方法
1.2.1. 胶体球模板制备
1.2.2. MnCo2O4/Ni纳米片复合多孔阵列电极的制备
1.2.3. 电化学测试
-
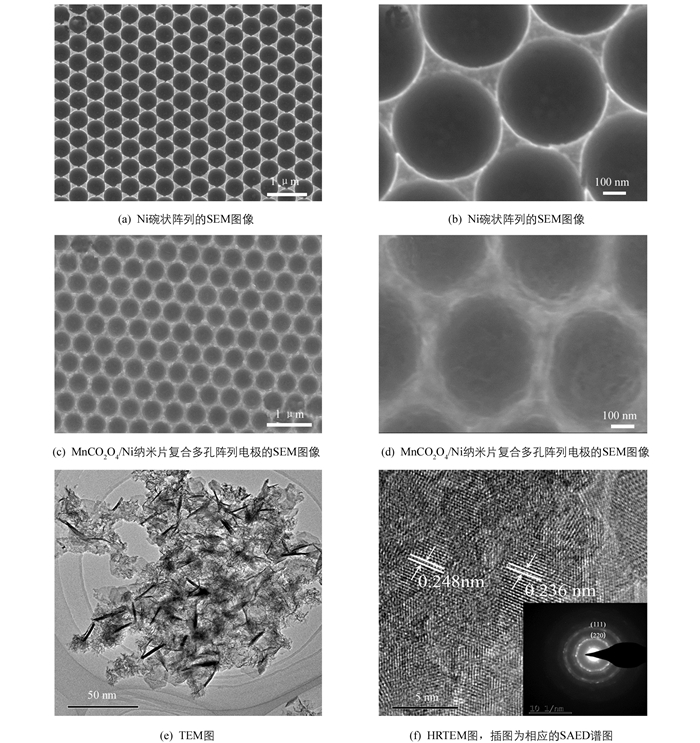
用SEM对MnCo2O4/Ni电极的形貌进行表征. 如图 2(a)和图 2(b)所示,采用恒电流电沉积法制备的Ni碗状阵列模板表面光滑,高度有序. 从图 2(c)和图 2(d)中可以看到有序的Ni碗状纳米阵列表面覆盖了一层纳米片,这些纳米片彼此交错生长,形成三维网状结构,使得所合成材料的比表面积增加,提供了大量的活性位点,有利于提升电极的电化学性能. 利用TEM进一步表征了MnCo2O4纳米片的形貌,如图 2(e). 图 2(f)的HRTEM图显示晶格间距为0.236 nm和0.248 nm的晶格条纹,分别对应MnCo2O4的(222)晶面和(311)晶面,图 2(f)的插图显示为清晰环的SAED图案,表明MnCo2O4纳米片具有多晶结构.
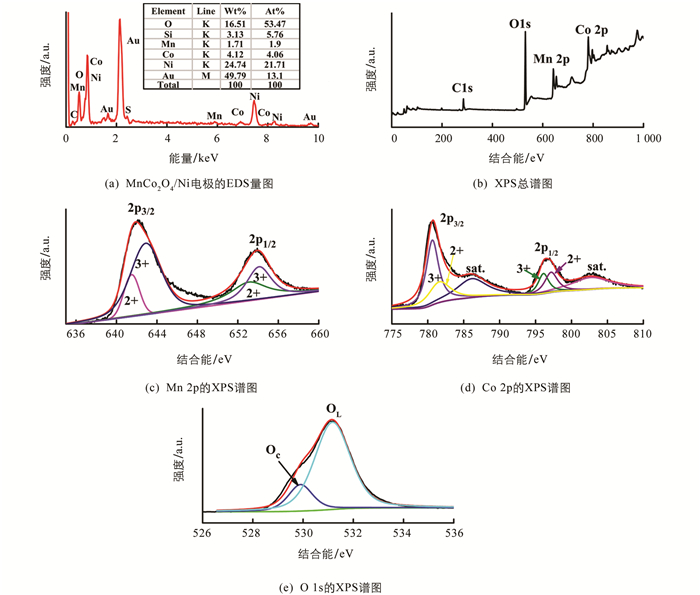
利用能谱仪对MnCo2O4/Ni分级微纳阵列进行了原位分析,图 3(a)显示产物由Mn,Co,O和S元素组成(其中Au,Si和部分O元素来自导电基底层和空气). 为了获得更详细的结构表面元素组成和价态信息,采用XPS对制备的材料进行分析,XPS总谱如图 3(b)所示. 相应Mn 2p谱有两个特征峰和一个卫星峰,如图 3(c)特征峰2p3/2(642.1 eV)和2p1/2(653.6 eV)之间的结合能差值为11.5 eV,表明存在着Mn2+和Mn3+,经过精细拟合后,位于641.5 eV和653.1 eV的特征峰表示存在Mn2+,位于642.9 eV和653.7 eV处的特征峰为Mn3+[20, 23, 27]. 图 3(d)中Co 2p的XPS谱表示Co 2p1/2和Co 2p3/2的特征峰,分别在780.6 eV和796.5 eV处,与文献[28]报道的结果一致. 此外,Co 2p1/2和Co 2p3/2的特征峰之间的结合能之差为16.5 eV,这表明同时存在Co2+和Co3+,位于781.6 eV和797.1 eV的特征峰表示存在Co2+,位于780.6 eV和796.0 eV处的特征峰表示存在Co 3+[29-30]. 对于图 3(e)中O 1s的XPS谱图,结合能529.9 eV和531.3 eV处的两个峰表示典型的晶格氧(OL)和吸附氧(OC)[23].
-
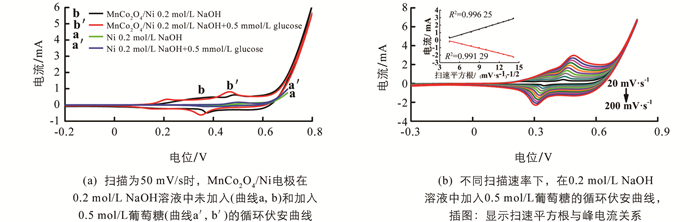
采用三电极体系对制备的MnCo2O4/Ni分级微纳阵列电极的电化学性能进行评估. 通过前期对实验参数的优化,我们选择0.2 mol/L NaOH溶液作为电解液进行测试,利用循环伏安法测试样品对葡萄糖的电化学响应,测试结果如图 4所示. 图 4(a)的曲线为0.2 mol/L NaOH溶液中在-0.3~0.8 V的电位区间内,扫描速度为50 mV/s时,不添加(曲线a,b)和加入0.5 mmol/L葡萄糖溶液(曲线a′,b′)后Ni碗状阵列电极和MnCo2O4/Ni分级微纳阵列电极的循环伏安曲线对比图. 通过对比可以发现,添加葡萄糖后,Ni碗状阵列电极(曲线a′)没有明显的氧化还原反应峰,MnCo2O4/Ni阵列电极(曲线b′)的电流值有所增加且在电位为+0.15V/+0.2V和+0.35V/+0.45V处出现两对氧化还原峰,这些峰各自对应Co和Mn的氧化还原对[31-33],说明MnCo2O4/Ni电极对葡萄糖具有良好的电催化活性,MnCo2O4/Ni电极在添加葡萄糖溶液后发生的电化学反应可以用式(1)和式(2)描述[33-37]:
在0.2 mol/L NaOH溶液中加入0.5 mmol/L葡萄糖后,研究MnCo2O4/Ni阵列电极在20~200 mV/s扫描速率对葡萄糖氧化的影响. 从图 4(b)中可以明显分析出随着扫描速率的增加,阳极峰出现显著的正位移,阴极峰出现明显的负位移,这可能是由于氧化还原过程中的电极极化增强所致. 此外,插图显示阳极和阴极峰值电流均与扫描速率的平方根线性相关,说明葡萄糖的氧化还原反应是扩散控制过程.
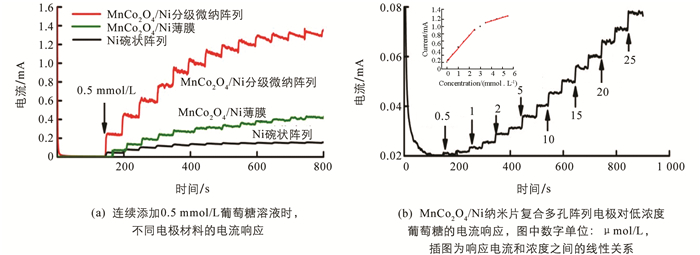
传感器的灵敏度、线性范围和检测极限是传感器不可缺少的性能参数. 图 5(a)展示了Ni阵列电极、MnCo2O4/Ni复合平面电极和MnCo2O4/Ni分级微纳阵列电极在电位为0.45 V时,在连续搅拌的0.2 mol/L氢氧化钠溶液中每隔50 s添加0.5 mmol/L葡萄糖溶液的电流-时间响应图. 结果表明,Ni碗状阵列电极对葡萄糖溶液几乎无响应. 与MnCo2O4/Ni纳米片复合平面电极相比,MnCo2O4/Ni纳米片复合多孔阵列电极具有更好的催化性能,这可能是由于分级微纳结构增大了电极的比表面积,增加了电化学活性表面位点,从而提升了MnCo2O4/Ni阵列电极的传感性能. 另一方面,MnCo2O4/Ni阵列电极对葡萄糖的电流响应显著提升,这可以归因于过渡金属尖晶石氧化物独特的电子结构能够促进溶液中电子的转移和锰-钴氧化物组分之间存在的协同作用能够促进电极对葡萄糖的电催化氧化反应[22, 34],从而显著提升了MnCo2O4/Ni阵列电极的传感器性能.
图 5(b)显示了连续添加不同小浓度的葡萄糖溶液时,MnCo2O4/Ni纳米片复合多孔阵列电极的响应电流快速增强且达到稳定值的时间小于3 s. 通过计算得到该电极的检测极限为0. 17 μmol/L,表明该电极对葡萄糖有良好的催化性能. 图 5(b)插图显示了电流与葡萄糖浓度的线性关系,从插图中可以看出响应电流与浓度的线性回归方程分别为:
根据公式(3),MnCo2O4/Ni分级微纳阵列电极在0.5 μmol/L~2.5 mmol/L葡萄糖浓度范围内,灵敏度为1 439.3 μA/[(mmol/L)·cm2]. 结合公式(4),MnCo2O4/Ni分级微纳阵列电极在3.5~5.5 mmol/L葡萄糖浓度范围内,灵敏度为309.4 μA/[(mmol/L)·cm2]. 表 1列出了部分以往报道的无酶葡萄糖传感器的性能,相比之下,本文制备的MnCo2O4/Ni纳米片复合多孔阵列电极具有更好的灵敏度、更宽的线性范围.
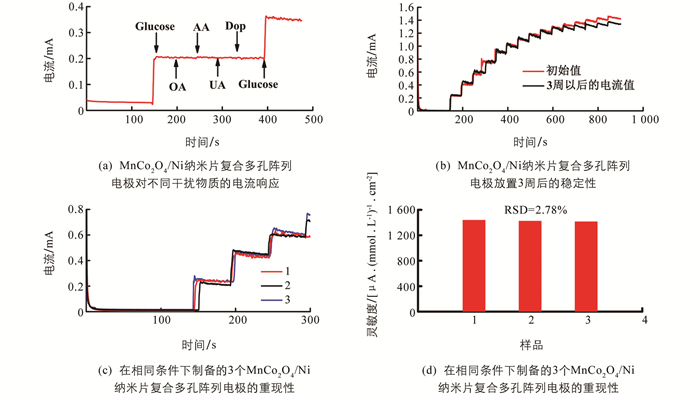
抗干扰性能也是无酶电化学传感器的重要参数. 在人体生理环境中,正常血糖水平的浓度明显高于血液中的AA,DA,UA和OA等诸多干扰物质. 采用与电流响应相同的实验条件,选择葡萄糖浓度为0.5 mmol/L,其他干扰物质的浓度均为0.05 mmol/L,对MnCo2O4/Ni分级微纳阵列电极进行抗干扰性能测试. 测试结果表明,在电解液中加入0.5 mmol/L葡萄糖溶液后,响应电流快速增大,而加入其他抗干扰物质几乎不会引起电流的变化,再次添加葡萄糖溶液,电流又发生了明显的阶跃,这表明MnCo2O4/Ni阵列电极具有良好选择性,如图 6(a).
此外,采用三电极体系,使用计时电流法在0.2 mol/L NaOH溶液中加入0.5 mmol/L葡萄糖溶液,测定该葡萄糖生物传感器电极的稳定性和重现性,如图 6(b)和图 6(c),结果表明,3周后电极对葡萄糖的响应仍然保持了96.8%的初始值,由此证明MnCo2O4/Ni纳米片复合多孔阵列电极具有优异的稳定性. 采用相同的方法分别制备了3个相同的MnCo2O4/Ni纳米片复合多孔阵列电极,结果显示标准差(RSD)为2.78%,证明该传感器具有良好的重现性,如图 6(d).
考虑到实际应用,利用制备的MnCo2O4/Ni纳米片复合多孔阵列电极,以市场上浓度为50%的葡萄糖注射液作为实际样品进行分析研究. 将葡萄糖注射液稀释100倍,向稀释后的葡萄糖注射液中分别加入0.2,0.4,0.5 mmol/L的葡萄糖标准溶液,MnCo2O4/Ni纳米片复合多孔阵列电极的测定结果如表 2,葡萄糖的回收率为97.12%~104.35%,表明此传感器可用于葡萄糖注射液的实际测定.
2.1. MnCo2O4/Ni纳米片复合多孔阵列电极的表征
2.2. MnCo2O4/Ni阵列电极的电化学性能
-
本文采用胶体球模板法和两步电沉积法成功制备了MnCo2O4/Ni分级微纳阵列电极,与复合平面电极相比,MnCo2O4/Ni分级微纳阵列结构提供了更大的比表面积,丰富的孔结构增强了电子的扩散速率;此外,Mn和Co的协同作用增强了反应过程的电子传输速度,从而提高了其对葡萄糖的电催化活性. 因此,MnCo2O4/Ni分级微纳阵列电极具有灵敏度高、选择性好、稳定性优异、检出限低等优点. 总之,本研究可为无酶葡萄糖传感器的电极材料研究提供参考.






 DownLoad:
DownLoad: