-
开放科学(资源服务)标志码(OSID):

-
浮游植物处于水生生态系统食物链的最底端,是许多水生动物的主要食物来源,它们的丰度和群落组成直接影响水生生态系统的平衡[1-2]. 若水体中浮游植物的数量和种类过少,水生生态系统的物种多样性会下降,抗干扰能力会降低;若水体中浮游植物的数量过多,其群落结构过于单一,水体可能会发生水华,对水生生态系统及人类生活产生一系列的负面影响,如导致水体溶解氧降低[3]、水生生态系统食物链和食物网被破坏[4]、减少水生生态系统物种多样性[5-6]、产生对消费者有毒的次生代谢产物[7-8]等,因此,浮游植物的数量和物种组成直接影响着水生生态系统的物种多样性和功能稳定性.
随着季节的自然更替,水体中各种环境因子也在不断发生变化,如水温、光照、pH值、营养盐、溶解氧等[9],水体中不同种类的浮游植物生长状况也在不断发生变化. 大量研究表明,在春、秋季,硅藻常常成为水体中的优势种,而在夏季,蓝藻和绿藻往往占据主导地位[10-13],因此,在同一生境中,水体中的浮游植物群落结构可能会随着季节的变化而变化.
三峡库区位于中国长江上游下段,拥有丰富的物种资源,被认为是中国最重要的生物多样性研究热点之一[14]. 由于人为活动的影响如修建大坝、污水排放等,三峡库区的生物多样性受到严重影响[15-17]. 三峡库区水体生物多样性的改变会对长江上游的生物多样性造成一定的影响. 研究发现,三峡大坝的修建导致三峡库区水体流速变慢,进而使三峡库区支流和干流一些喜流水性的鱼类被迫向长江上游迁徙,导致长江上游的鱼类群落物种组成和功能类群发生改变[18],因此,保护三峡库区水体生物多样性对维持整个长江生物多样性具有重要意义. 浮游植物作为三峡库区水生生态系统中最重要的初级生产者,对维持三峡库区水体生物多样性具有重要作用. 三峡库区长江干流浮游植物物种多样性直接受到支流浮游植物物种多样性的影响,为了保护三峡库区水体物种多样性,了解支流浮游植物群落物种多样性变化就显得尤为重要.
三峡库区分布着许多大大小小的支流,其中一级支流有38条[19]. 不同支流所处的地理位置以及人为干扰程度不同,因此,每条支流为浮游植物提供的生长环境条件也不尽相同,如流速、水温、水体浊度等,导致不同支流的浮游植物群落可能存在一定的差异. 目前,关于三峡库区不同支流浮游植物群落比较的研究较少. 本研究选取了位于三峡库区北岸的一级支流汝溪河和位于三峡库区南岸的一级支流龙河作为研究对象,拟探究三峡库区不同支流浮游植物群落季节动态是否存在差异,为保护三峡库区水体物种多样性提供一定的理论依据.
HTML
-
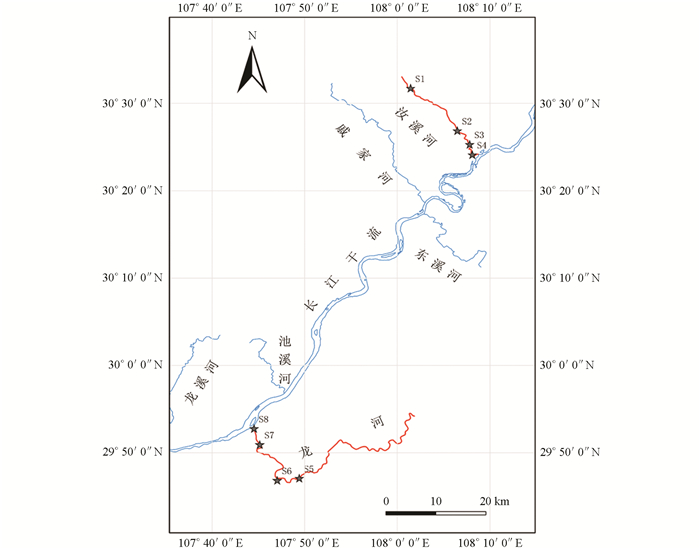
汝溪河位于三峡库区北岸,发源于万州区分水镇三角凼,流经梁平区与右支流汇合后,最后经忠县涂井乡汇入长江. 该河主河道长54.5 km,流域面积720 km2,其中,忠县境内主河道长25.4 km,流域面积272.9 km2,年平均流量为4.72 m3/s. 忠县属于亚热带湿润季风气候,年平均气温18.2 ℃,年均降雨量1 200 mm. 龙河位于三峡库区南岸,发源于鄂渝交界处,是石柱县、丰都县境内最大的河流. 龙河干流全长164 km,流域面积2 779 km2. 龙河(丰都段)河流长62.5 km,流域面积约1 348 km2,年平均流量为64.9 m3/s. 丰都县年平均气温18.0 ℃,年均降雨量1 229 mm. 本研究中每条支流从上游至下游分别设置4个采样断面,其中汝溪河从上游至下游设置的4个采样断面分别为白庙(S1)、老涂井(S2)、龙滩大桥(S3)、汝溪河入江口(S4);龙河从上游至下游设置的4个采样断面分别为九溪沟大桥(S5)、下刀弓溪(S6)、两会口(S7)、龙河入江口(S8)(图 1). 本研究分别于2021年春季(3月)、夏季(7月)、秋季(9月)以及冬季(11月)开展野外调查研究工作.
-
样品采集方法参照《水环境监测规范》(SL219-2013),每个断面使用5 L的采水器分别采集左岸、中泓和右岸表层0.5 m处水样,采集的水样分别装入550 mL和400 mL采样瓶中,带回实验室用于营养盐指标的测定. 用25#浮游生物采集网(网孔0.064 mm)于表层至0.5 m处划“∞”来回拖拽数次,将采集的浮游植物装入50 mL的采样瓶中,并立即加入1.5 mL鲁哥试剂进行固定,用于浮游植物定性鉴定;同时,采集1 L的表层水体装入采样瓶中,并立即加入15 mL鲁哥试剂现场固定,带回实验室浓缩至30 mL左右,用于浮游植物定量鉴定. 将采集的浮游植物带回实验室于显微镜下进行物种鉴定并计数. 本研究主要参照《中国淡水藻类》[20]《中国淡水藻类:系统、分类及生态》[21]《福建省大中型水库常见淡水藻类图集》[22] 《中国内陆水域常见藻类图谱》[23]等进行浮游植物的物种鉴定.
-
水环境因子指标包括水温(WT),pH值,氧化还原电位(ORP),电导率(EC),浊度(Tur),溶解氧(DO),流速(V),透明度(SD),高锰酸盐指数(CODMn),总氮(TN),硝态氮(NO3--N),氨氮(NH4+-N),总磷(TP),磷酸盐(PO43--P),总碱度(CaCO3),叶绿素a(Chl a). WT,pH值,ORP,EC,Tur,DO使用DS5X多参数水质测定仪现场测定;V使用国产LS45A型旋杯式流速仪测量(华正水文仪器有限公司,重庆);SD使用赛氏圆盘现场测定;CODMn,TN,NO3--N,NH4+-N,TP,PO43-P,CaCO3,根据《水和废水监测分析方法》进行检测;叶绿素a质量分数根据《水质叶绿素a的测定分光光度法》测定.
-
采用丰度、优势度(Y)、Shannon-Wiener多样性指数(H)、Margalef丰富度指数(D)和Pielou均匀度指数(J)对汝溪河和龙河浮游植物群落结构进行分析,各指标计算公式:
式中,ni为样品中第i种浮游植物的个体数,N为样品中浮游植物总个体数,fi为第i种浮游植物在各样点出现的频率,S为浮游植物种类数.
-
采用Microsoft Excel 2016,Origin 2021和SPSS 25.0对环境因子指标和浮游植物物种数、丰度、优势种以及多样性指数进行相关统计分析和作图.
采用Pearson相关性分析浮游植物总丰度、优势藻门丰度、多样性指数与环境因子之间的关系.
采用典范排序分析汝溪河和龙河浮游植物群落与环境因子之间的相关性,用于排序分析的种群为汝溪河和龙河浮游植物相对丰度大于2%的物种. 根据去趋势对应分析(Detrended Correspondence Analysis,DCA)结果,选择基于线性模型的冗余分析(Redundancy Analysis,RDA). 由于环境变量之间可能存在较高的相关性,首先将所有环境因子作为解释变量纳入RDA,查看其方差膨胀因子(Variance Inflation Factor,VIF)大小,一般认为VIF大于20时,变量共线性强,需对环境因子进行筛选. 采用前向选择法通过Monte Carlo检验(p<0.01,n=999)对变量进行筛选. 通过筛选,用于汝溪河浮游植物群落结构RDA的环境因子为WT,pH值,DO,EC,Tur,SD,TN和TP,用于龙河浮游植物群落结构RDA的环境因子为WT,EC,Tur,V和ORP. 以上所有统计分析均使用R语言(4.0.3版)进行.
1.1. 研究支流概况及断面设置
1.2. 样品采集和处理
1.3. 水环境因子指标测定
1.4. 数据分析
1.4.1. 浮游植物群落结构分析
1.4.2. 数据统计分析
-
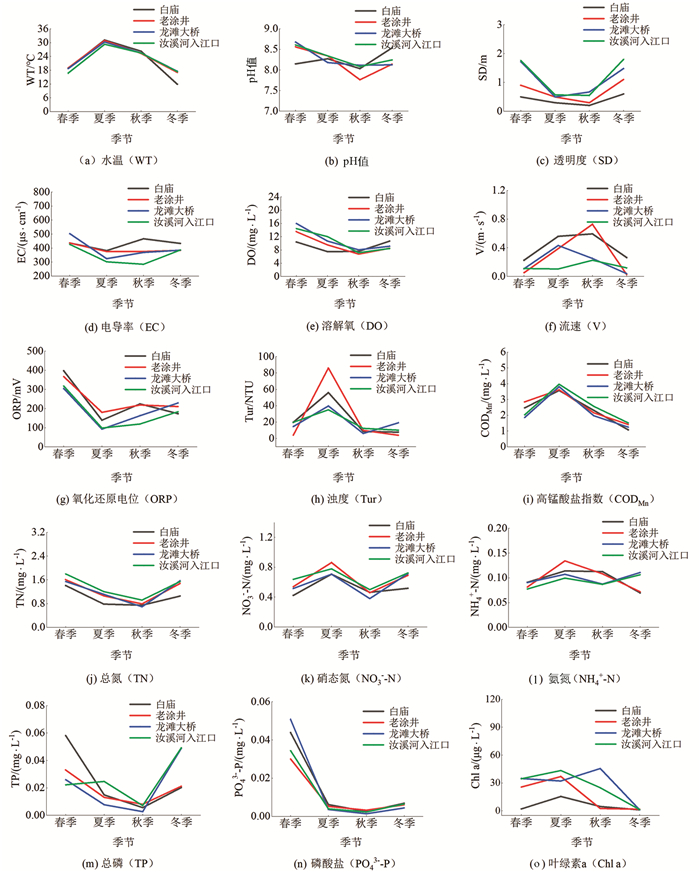
汝溪河不同季节水体理化因子特征见图 2.
在同一季节,汝溪河4个断面的水温基本相同,其中,夏季水温最高,为30.40±0.41 ℃,冬季水温最低,为15.95±1.36 ℃;汝溪河不同季节各断面水体pH值变化范围为7.76~8.68,水体整体呈碱性;透明度变化范围为0.20~1.80 m,春季和冬季水体透明度较高;浊度变化范围为3.90~86.00 NTU,夏季水体浊度较高,平均值为54.17 NTU;溶解氧浓度变化范围为6.78~15.97 mg/L,各断面春季水体溶解氧均较高;高锰酸盐指数与水温呈现出相同的季节变化趋势,变化范围为1.07~3.97 mg/L,夏季水体高锰酸盐指数较高,平均值为3.75 mg/L;TN,TP质量浓度变化范围分别为0.69~1.80 mg/L,0.003~0.060 mg/L,春季TN,TP平均质量浓度分别为1.59 mg/L,0.040 mg/L;叶绿素a质量浓度平均为19.25 μg/L.
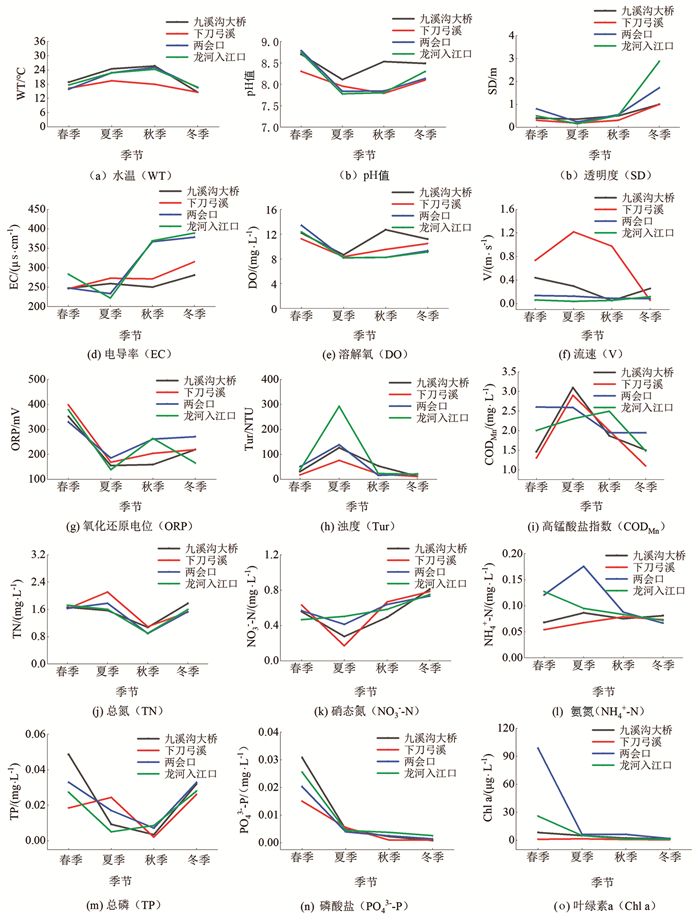
龙河不同季节水体理化因子特征见图 3. 各断面水温变化范围为14.59~25.67 ℃,夏季水温较高,冬季水温较低;pH值变化范围为7.78~8.79,水体整体呈碱性;透明度变化范围为0.15~2.88 m,春季和冬季水体透明度较高;浊度变化范围为11.20~292.57 NTU,夏季水体浊度较高,平均值为158.28 NTU;溶解氧浓度变化范围为8.16~13.40 mg/L,春季水体溶解氧较高;高锰酸盐指数变化范围为1.10~3.10 mg/L,夏季水体高锰酸盐指数较高,平均值为2.72 mg/L;TN,TP质量浓度变化范围分别为0.89~2.11 mg/L,0.002~0.050 mg/L,春季TN,TP平均质量浓度分别为1.66 mg/L,0.030 mg/L;叶绿素a质量浓度平均为10.44 μg/L.
-
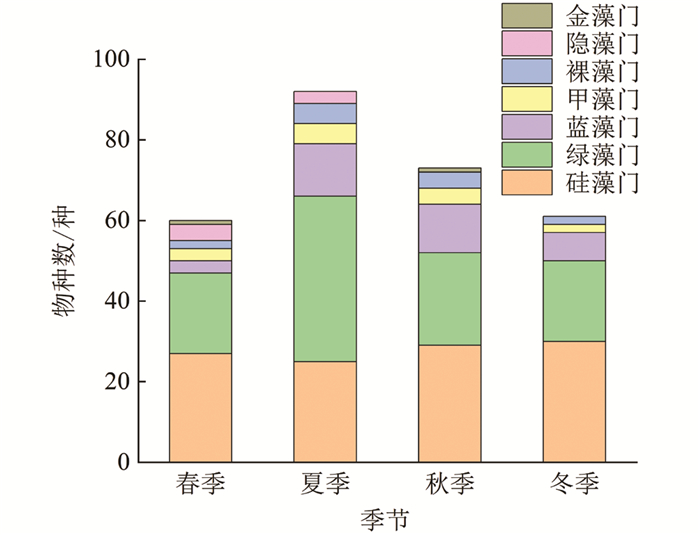
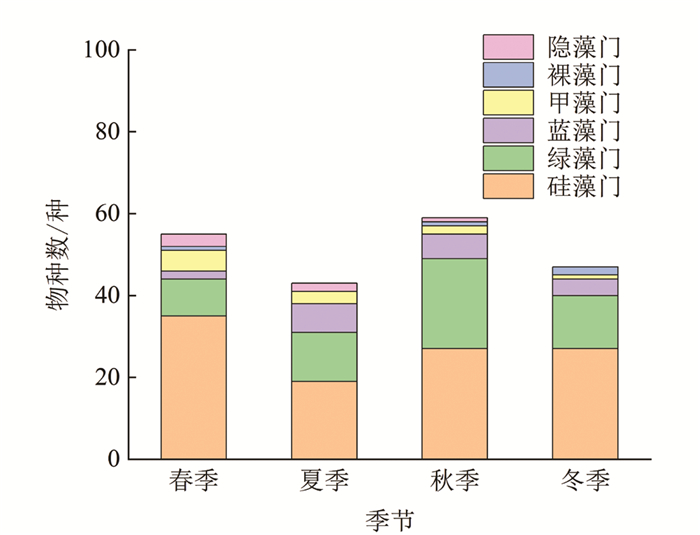
本研究共鉴定出汝溪河中有浮游植物7门139种(图 4),其中,硅藻门49种,占浮游植物种类数的35.3%;绿藻门58种,占41.7%;蓝藻门15种,占10.8%;甲藻门6种,占4.3%;裸藻门6种,占4.3%;隐藻门4种,占2.9%;金藻门1种,占0.7%. 通过比较不同季节浮游植物的变化可以发现,夏季浮游植物物种数较多,除夏季外,其他季节均以硅藻门物种数占据优势,其次为绿藻门和蓝藻门. 从不同优势门类来看,硅藻门浮游植物物种数在冬季最多,达30种,夏季最少,仅25种;绿藻门浮游植物物种数在夏季最多,达41种,春季和冬季最少,仅20种;蓝藻门浮游植物物种数在夏季最多,达13种,春季最少,仅3种.
本研究共鉴定出龙河中有浮游植物6门108种(图 5),其中,硅藻门50种,占浮游植物种类数的46.3%;绿藻门36种,占33.3%;蓝藻门10种,占9.3%;甲藻门7种,占6.5%;裸藻门2种,占1.9%;隐藻门3种,占2.7%. 通过比较不同季节浮游植物的变化发现,春、秋两季浮游植物物种数较多,且不同季节均以硅藻门物种数占据优势,其次为绿藻门和蓝藻门. 从不同优势门类来看,硅藻门浮游植物物种数在春季最多,达35种,夏季最少,仅19种;绿藻门浮游植物物种数在秋季最多,达22种,春季最少,仅9种;蓝藻门浮游植物物种数在夏季最多,达7种,春季最少,仅2种.
-
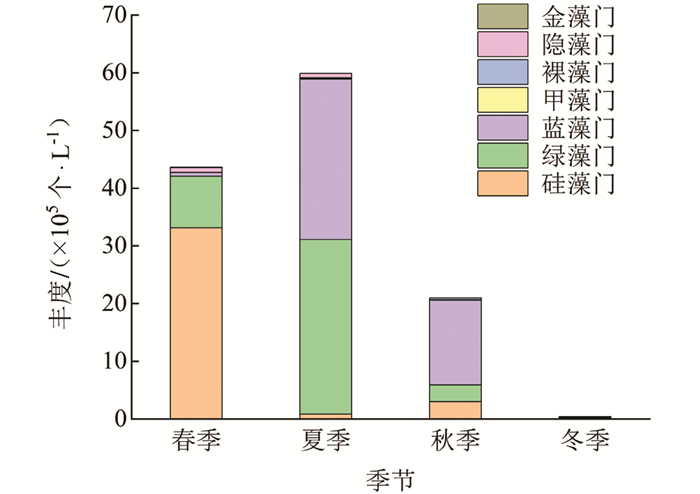
调查期间,汝溪河不同季节浮游植物丰度变动范围为0.44×105~59.87×105个/L,整体上丰度变动由大到小依次为夏季、春季、秋季、冬季(图 6). 汝溪河浮游植物群落以硅藻门、蓝藻门、绿藻门为主. 汝溪河不同季节浮游植物群落丰度存在一定的差异,其中,春季浮游植物丰度主要由硅藻门贡献,占浮游植物总丰度的76%;夏季浮游植物丰度主要由蓝藻门和绿藻门贡献,分别占浮游植物总丰度的46%和51%;秋季浮游植物丰度主要由蓝藻门贡献,占浮游植物总丰度的70%;冬季浮游植物丰度主要由硅藻门贡献,占浮游植物总丰度的39%. 硅藻门相对丰度在春季达到最高,为76%;蓝藻门相对丰度在秋季达到最高,为70%,绿藻门相对丰度在夏季达到最高,为51%.
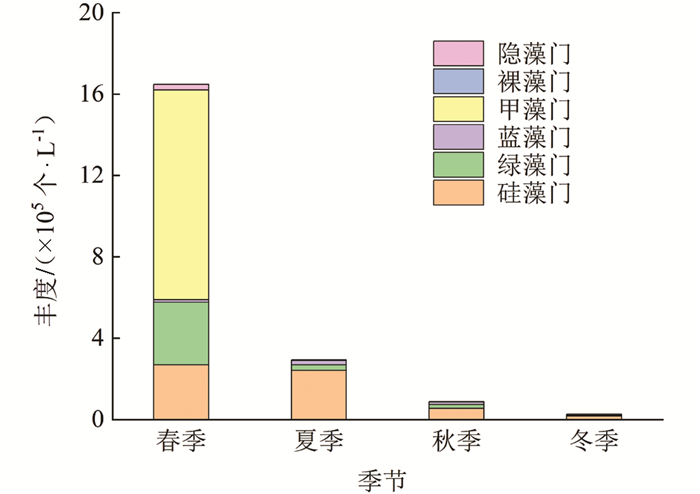
调查期间,龙河不同季节浮游植物丰度变动范围为0.28×105~16.49×105个/L,整体上丰度变动由大到小依次为春季、夏季、秋季、冬季(图 7). 龙河浮游植物群落以硅藻门、甲藻门为主. 龙河不同季节浮游植物群落丰度存在一定的差异,其中,春季浮游植物丰度主要由甲藻门贡献,占总丰度的63%;夏季、秋季、冬季浮游植物丰度均主要由硅藻门贡献,分别占总丰度的82%,63%和65%;甲藻门相对丰度在春季达到最高,为63%;硅藻门相对丰度在夏季达到最高,为82%.
-
汝溪河共有优势种11种,其中硅藻门2种、绿藻门6种、蓝藻门2种、裸藻门1种(表 1).
不同季节优势种组成明显不同,春季优势种以硅藻门和绿藻门为主,意大利直链藻(Melosira italica)优势度最大;夏季、秋季及冬季优势种均以蓝藻门和绿藻门为主,假鱼腥藻(Pseudoanabaena sp.)优势度最大.
龙河共有优势种26种,其中硅藻门13种、绿藻门9种、蓝藻门3种、甲藻门1种(表 2). 不同季节优势种组成明显不同,春季优势种以甲藻门、硅藻门和绿藻门为主,倪氏拟多甲藻(Peridiniopsis sp.)优势度最大;夏季优势种以硅藻门和蓝藻门为主,尖针杆藻(Synedra acus)优势度最大;秋季优势种主要以硅藻门、蓝藻门和绿藻门为主,变异直链藻(Melosira varians)优势度最大;冬季优势种主要以硅藻门为主.
-
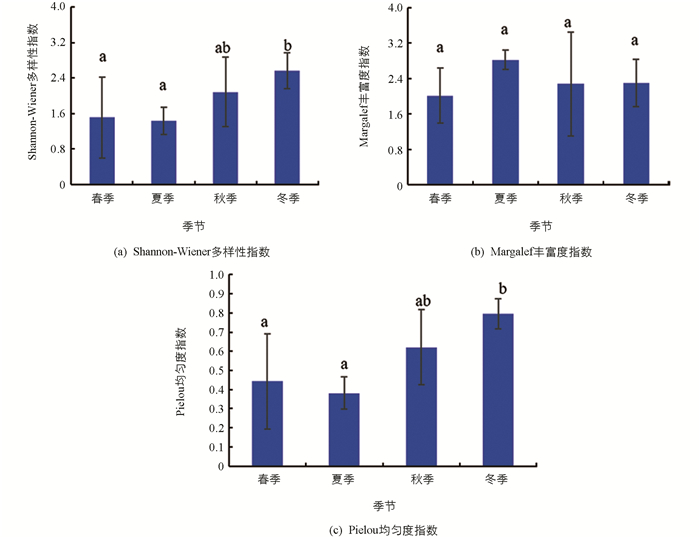
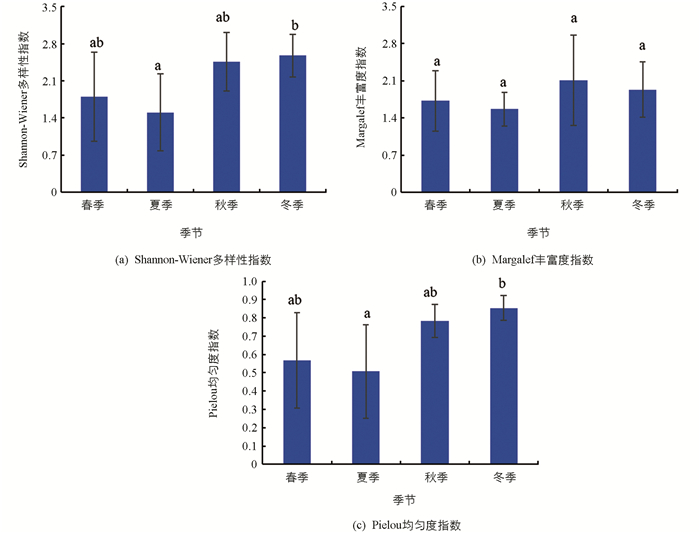
汝溪河不同季节浮游植物Shannon-Wiener多样性指数变化范围为1.44~2.57,均值为1.90;Margalef丰富度指数变化范围为2.01~2.82,均值为2.35;Pielou均匀度指数变化范围为0.38~0.79,均值为0.56(图 8). Shannon-Wiener多样性指数在冬季显著高于春季和夏季(p<0.05),与秋季差异无统计学意义(p>0.05);Margalef丰富度指数在不同季节之间差异无统计学意义(p>0.05);Pielou均匀度指数在冬季显著高于春季和夏季(p<0.05),与秋季差异无统计学意义(p>0.05).
龙河不同季节浮游植物Shannon-Wiener多样性指数变化范围为1.50~2.57,均值为2.08;Margalef丰富度指数变化范围为1.56~2.11,均值为1.83;Pielou均匀度指数变化范围为0.51~0.85,均值为0.68(图 9). Shannon-Wiener多样性指数在冬季显著高于夏季(p<0.05),与春季和秋季差异无统计学意义(p>0.05);Margalef丰富度指数在不同季节之间差异无统计学意义(p>0.05);Pielou均匀度指数在冬季显著高于夏季(p<0.05),与春季和秋季差异无统计学意义(p>0.05).
-
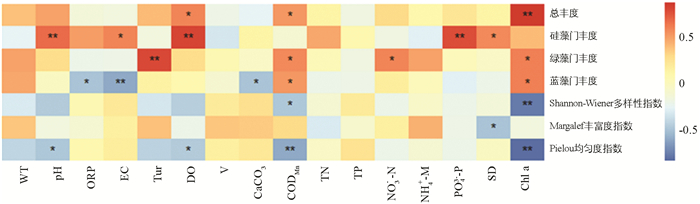
汝溪河浮游植物总丰度、优势藻门丰度和多样性指数与环境因子的Pearson相关系数分析见图 10. 结果表明:汝溪河浮游植物总丰度与DO和CODMn呈显著正相关;硅藻门丰度与pH值,DO和PO43--P呈极显著正相关,与EC和SD呈显著正相关;绿藻门丰度与Tur呈极显著正相关,与CODMn和NO3--N呈显著正相关;蓝藻门丰度仅与CODMn呈显著正相关,与ORP,EC和CaCO3呈显著或极显著负相关;Shannon-Wiener多样性指数与CODMn呈显著负相关;Margalef丰富度指数仅与SD呈显著负相关,与其他环境因子相关性不大;Pielou均匀度指数与pH值,DO和CODMn呈显著或极显著负相关;Chl a与浮游植物总丰度呈极显著正相关.
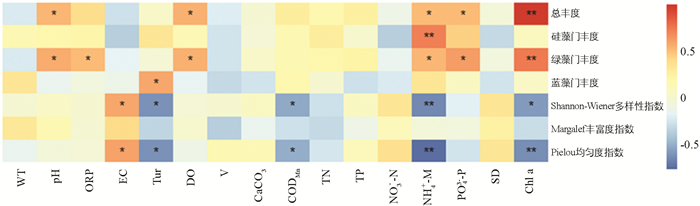
龙河浮游植物总丰度、优势藻门丰度和多样性指数与环境因子的Pearson相关系数分析见图 11. 结果表明:龙河浮游植物总丰度与pH值,DO,NH4+-N和PO43--P呈显著正相关;硅藻门丰度仅与NH4+-N呈极显著正相关;绿藻门丰度与pH值,ORP,DO,NH4+-N和PO43--P呈显著正相关;蓝藻门丰度仅与Tur呈显著正相关,与其他环境因子相关性不大;Shannon-Wiener多样性指数和Pielou均匀度指数均与EC呈显著正相关,与Tur,CODMn和NH4+-N呈显著或极显著负相关;Margalef丰富度指数与各水环境因子的相关性不大;Chl a与浮游植物总丰度、绿藻门丰度呈极显著正相关.
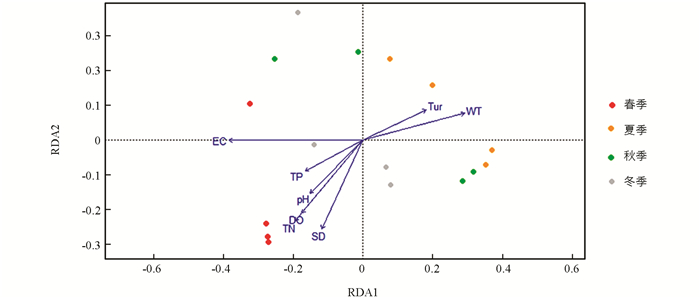
汝溪河不同季节浮游植物群落与环境因子的RDA结果见图 12. 结果表明,69.70%的总方差由环境变量约束轴解释,其中,第1轴和第2轴的特征值分别为0.193和0.119,占总方差的44.26%. 第1轴与WT呈显著正相关,与EC呈显著负相关(p<0.001);第2轴与SD呈显著负相关(p<0.001). 春季浮游植物群落主要受pH值,DO,EC,TN和TP的影响;夏季和秋季浮游植物群落主要受WT和Tur的影响;冬季浮游植物群落主要受SD的影响.
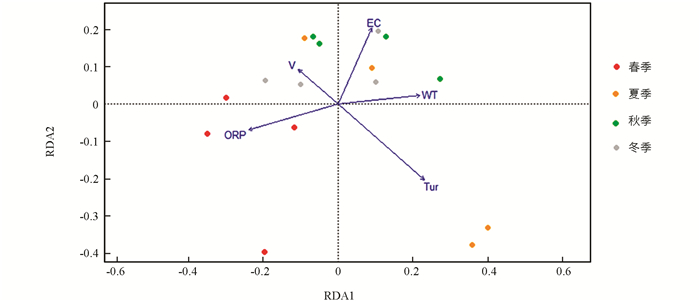
龙河不同季节浮游植物群落与环境因子的RDA结果见图 13. 结果表明,43.86%的总方差由环境变量约束轴解释,其中,第1轴和第2轴的特征值分别为0.108和0.083,占总方差的31.92%. 第1轴与WT呈显著正相关,与ORP呈显著负相关(p<0.001);第2轴与EC呈显著正相关(p<0.001). 春季浮游植物群落主要受ORP的影响;夏季浮游植物群落主要受Tur的影响;秋季和冬季浮游植物群落主要受WT,EC和V的影响.
2.1. 汝溪河和龙河不同季节水体理化因子特征
2.2. 汝溪河和龙河不同季节浮游植物物种组成
2.3. 汝溪河和龙河不同季节浮游植物丰度
2.4. 汝溪河和龙河不同季节浮游植物优势种
2.5. 汝溪河和龙河不同季节浮游植物多样性
2.6. 汝溪河和龙河浮游植物群落与环境因子的相关性
-
在本研究中,共鉴定出汝溪河硅藻门浮游植物49种,占浮游植物总物种数的35.3%,而龙河共鉴定出硅藻门浮游植物50种,占浮游植物总物种数的46.3%,表明龙河喜流水性的硅藻种类占比高于汝溪河,这可能与两条支流水体的流量和流速不同有关. 据调查显示,龙河年平均流量为64.9 m3/s,而汝溪河年平均流量仅为4.72 m3/s[24-25],因此,在具备高的水体流量和流速的水环境条件下,更有利于喜流水性浮游植物的生长和繁殖. 另外,本研究发现,汝溪河一年四季的浮游植物丰度显著高于龙河,这可能与汝溪河和龙河的地理位置不同有关. 汝溪河与龙河相比距离三峡大坝更近,汝溪河受到三峡大坝蓄水所引起的长江干流顶托作用更大. 大量研究表明,水库蓄水导致水体流速减慢,营养盐升高,众多支流开始出现水华现象[26-28]. 因此,与龙河相比,汝溪河水体的流速较慢,其营养盐更充足,更有利于浮游植物的生长. 除此之外,汝溪河优势种主要以蓝藻为主,因此,汝溪河更具有暴发水华的潜力. 对汝溪河和龙河的浮游植物群落物种多样性分析结果显示,龙河浮游植物Pielou均匀度指数大于汝溪河,表明龙河不同浮游植物物种个体数目分配均匀程度更大. 研究表明,三峡水库周期性蓄水导致不同支流水环境发生了不同程度的改变,进而使不同支流浮游植物物种呈现出不同的分布格局[12],因此,水库运行及其周期性蓄水倒灌引起的不同支流水文情势、栖息生境、水生生物等的差异均会对不同支流浮游植物群落物种分布产生不同的影响.
-
水体溶解氧通常会影响水生生物的细胞呼吸和生长代谢[29]. 本研究Pearson相关分析结果表明,DO对龙河和汝溪河浮游植物群落结构均具有显著影响,其他研究结果与本研究结果一致[30]. 水体浊度会影响水体中的光照强度,进而影响浮游植物进行光合作用[31]. 本研究发现,龙河水体Shannon-Wiener多样性指数和Pielou均匀度指数均与Tur呈显著负相关,而汝溪河水体Shannon-Wiener多样性指数和Pielou均匀度指数与Tur相关性不大. 由于龙河水体平均浊度显著高于汝溪河,水体浊度对龙河浮游植物群落结构影响更大. 高锰酸盐指数是反映水体受到有机污染物和还原性无机污染物污染程度的综合指标[32]. 本研究发现,汝溪河水体的平均高锰酸盐指数大于龙河,与龙河相比,汝溪河浮游植物丰度受到高锰酸盐指数的影响更大(图 10,图 11),表明汝溪河水体受到的污染程度更严重. RDA双序图结果显示,水温对汝溪河和龙河浮游植物群落结构均具有显著影响. 在适宜的温度范围内,水温升高有利于提高浮游植物的生长代谢速率,进而加速浮游植物的生长[33-34],但不同种类的浮游植物适宜生长的温度范围存在差异. 本研究发现,一年四季中,汝溪河和龙河的蓝藻门浮游植物物种数均在夏季最多,这可能与蓝藻更喜高温有关[35],因此,汝溪河和龙河的浮游植物与水温的相关性在夏季、秋季较高(图 12,图 13).
根据本文研究结果发现,相较于龙河,汝溪河更具有暴发水华的潜质,因此,应重点关注汝溪河的水质状况,加大对汝溪河的水质监测力度. 为防止汝溪河水华的发生,需要对水体营养盐和污染物进行有效控制,以降低水体的综合营养状态.






 DownLoad:
DownLoad: