-
开放科学(资源服务)标识码(OSID):

-
当今社会面临环境恶化和能源短缺问题,可持续的能源转换已引起人们密切关注,设计简单经济、绿色环保的能源转换技术迫在眉睫[1-3]. 电化学分解水是一种快速、绿色、高效的制氢技术,可以替代传统的化石燃料[4-6]. 水分解反应的两个半反应分别是析氢反应(HER)和析氧反应(OER),其中OER涉及多电子转移过程而致反应动力学迟缓,严重限制了制氢的效率[7]. 贵金属催化剂,如IrO2和RuO2,在催化OER方面表现出优异的性能,但是贵金属的稀缺性和高成本限制了它的实际应用[8]. 因此,开发高效、易获得、低成本的催化剂迫在眉睫.
过渡金属化合物,如过渡金属氧化物[9]、硫化物[10]、磷化物[11-12]等,是高效、易获得、低成本的水分解催化剂. 其中,过渡金属磷化物因其良好的导电性、高耐腐蚀性和优良的水分解电催化活性,具有潜在的应用前景. 在众多过渡金属磷化物中,镍基磷化物因具有活性位点丰富、成分可控、结构多样的特点受到广泛的关注. 但是,原始Ni2P的本征活性低,催化活性中心的密度小,限制了它在实际应用中的催化效率[13]. 阴离子或阳离子掺杂[14-15]、多孔纳米结构设计[16-17]、构造复合材料或异质结[18-19]、电化学活化[20-22]等是改善其催化活性的有效方法. 在这些方法中,阳离子掺杂通过增加催化位点、提高电子导电性和调节价态来提高催化活性已被广泛应用[23]. Zhao等[24]报道了金属V掺杂Ni2P催化剂的原位结构重建,在OER方面表现出优异的性能,在10 mA/cm2的电流密度下具有221 mV的过电位,且在碱性电解质中具有良好稳定性. Sun等[25]报道的杂化双零价非金属(N和B)掺杂的Ni2P,可以在全pH中进行HER,具有高活性和稳定性. 金属Ce具有比Ni更低的电负性,其掺杂可以显著调节Ni2P的电子结构,从而更有利改善催化剂的性能. 此外,金属Ce具有独特的4f电子结构,而且三价Ce和四价Ce还会相互灵活转化[26-27],有利于提升性能[28-29].
在本工作中,成功制备了Ce掺杂Ni2P纳米片催化剂Ce-Ni2P,且在1.0 M KOH中该催化剂表现出优异的OER性能. 在50和100 mA/cm2电流密度下,Ce-Ni2P的OER过电位分别为241和281 mV,显著优于Ni2P和商用RuO2的过电位. 此外,该催化剂展现出长期的OER稳定性.
HTML
-
将2 cm×3 cm的泡沫镍超声去除表面杂质. 1.2 mmol Ni(NO3)2·6H2O和0.096 mmol Ce(NO3)3·9H2O溶于20 mL去离子水中,搅拌得到均匀溶液. 预处理过的泡沫镍放置在这个均匀溶液里,并转移到1个50 mL的特氟龙内衬不锈钢高压釜中,在120 ℃加热6 h. 反应产物冷却到室温,用蒸馏水和乙醇冲洗黑色泡沫镍几次,真空干燥6 h. 得到的前驱体在管式炉里以2 ℃/min的升温速率升至350 ℃,并保持2 h,得到催化剂Ce-Ni2P/NF.
-
用D2 PHASER X射线衍射仪测量了制备样品的XRD数据. 在XL30型ESEM FEG扫描电子显微镜上进行了扫描电镜测试. 利用JEOL JEM 2100 F光谱仪采集了TEM和HRTEM图像. XPS在Thermo Scientific K-Alpha+上测量. 在室温下利用Bruker EMXnano光谱仪进行电子顺磁光谱EPR测量.
-
在CHI 660E电化学工作站上进行电化学测试. 在碱性条件下,分别以负载催化剂的NF(1 cm×1cm)、石墨棒和氧化汞(Hg/HgO)电极作为工作电极、计数电极和参比电极. 线性扫描伏安(LSV)曲线经过红外校正,扫描速率为5 mV/s. 在碱性双电极电解槽中,采用催化剂负载的NF作为双功能催化剂,评价了整体水裂解活性. 电化学阻抗谱(EIS)在100 kHz~1 mHz外加电位(1.43 V vs. RHE)下进行. 在不同扫描速率(60~100 mV/s)下利用循环伏安法(CV)得到的双层电容(Cdl)作为评估电化学活性表面积(ECSA)的指标.
1.1. Ce-Ni2P/NF的合成
1.2. 材料表征
1.3. 电化学测量
-
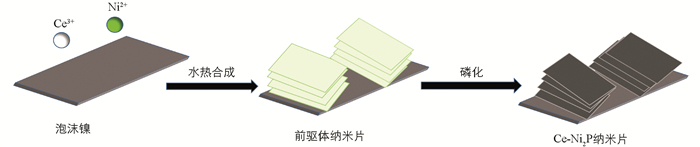
通过水热法和煅烧法合成了Ce-Ni2P/NF纳米片,制备过程见图 1.
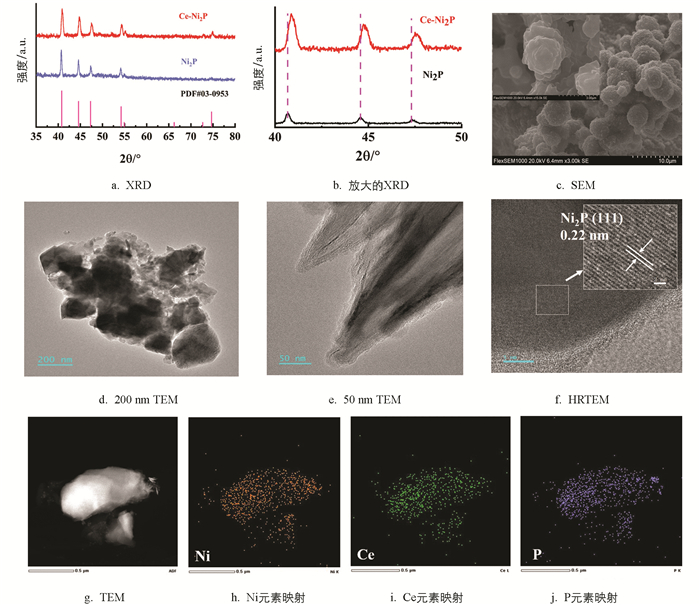
Ni2P的XRD衍射峰位于40.7°、44.5°、47.7°、54.2°、54.9°、66.2°、72.6°、74.6°(图 2a),分别对应(200)、(111)、(210)和(300)晶面,与Ni2P的标准谱(JCPDF NO.03-0953)一致,表面成功合成催化剂Ni2P. 值得注意的是,与原始的Ni2P相比,Ce-Ni2P样品的衍射峰略微向大角度方向位移(图 2b),这可能是因为空位的存在[30-31]. 扫描电镜(SEM)和透射电镜(TEM)图像表明(图 2c-2e),Ce-Ni2P具有堆叠的纳米片形貌. Ce-Ni2P表面的晶格间距为0.22 nm(图 2f),对应Ni2P的(111)面. 高角度环形暗场扫描TEM(HAADF-STEM)和EDX元素映射图像表明(图 2g-2j),Ni、Ce和P元素均匀分布在Ce-Ni2P催化剂中.
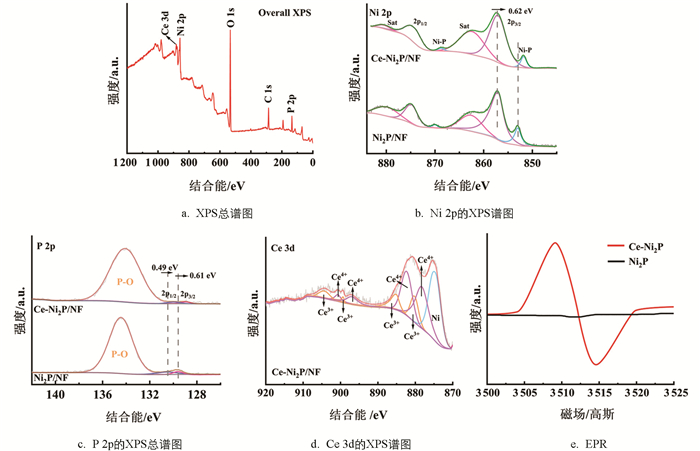
Ce-Ni2P的X射线光电子能谱(XPS)显示(图 3a),制备的样品含有Ce、Ni、P和O元素,其中O元素是由于样品在空气中被氧化所致,进一步表明Ce掺杂催化剂Ce-Ni2P的生成. 对于Ni2P的Ni 2p光电子能谱(图 3b),在870.02和852.99 eV结合能处的峰归属于Ni-P键[32],证明成功合成了Ni2P. 在857.04和874.83 eV处的峰分别归属于Ni 2p3/2和Ni 2p1/2,在860.89和879.86 eV处是2个卫星峰[33]. 在Ce-Ni2P催化剂中,Ni 2p1/2特征峰负位移0.62 eV的结合能较大,这意味着Ni的电子密度增加,化学价态降低. Ce-Ni2P中Ni 2p1/2特征峰位移明显,表明Ce掺杂能够显著调控催化剂的电子密度,从而极大地改善催化剂的固有活性. Ce掺杂导致催化剂中Ni的化学价态降低主要归因于掺杂诱导的磷空位(图 3e)[34]. 催化剂表面空位的产生能够显著改善OER催化活性. 对于P 2p光电子能谱(图 3c),Ce-Ni2P的P 2p1/2和P 2p3/2特征峰分别位于129.58和128.5 eV结合能处[35],并相对于Ni2P的相应特征峰负位移0.49和0.61 eV,这也归因于磷空位的存在. 图 3d中的Ce 3d光电子能谱分别含有4个归属于Ce4+的峰和4个归属于Ce3+的峰[36]. 催化剂中三价Ce和四价Ce相互转化能促进电荷的转移,提高催化剂的活性[37]. 电子顺磁光谱EPR表明(图 3e),Ce掺杂导致磷空位的产生.
-
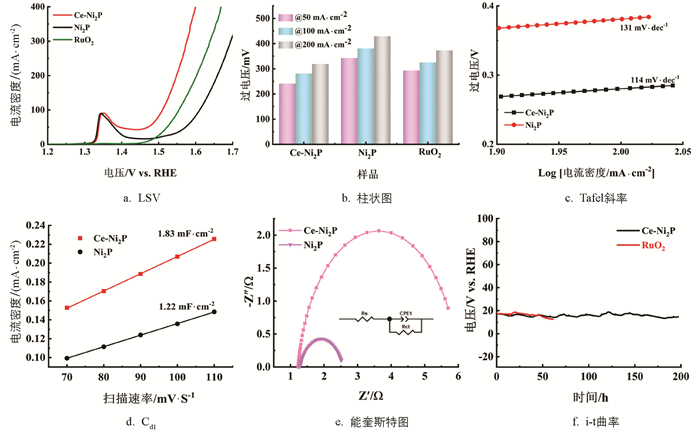
在1.0 M KOH条件下,评估了Ce-Ni2P/NF的催化活性. 如图 4a和图 4b所示,电流密度达到50 mA/cm2,Ce-Ni2P催化剂的OER过电位为241 mV,显著优于纯Ni2P的343 mV过电位和商用RuO2的293 mV过电位. 电流密度达到100 mA/cm2,Ce-Ni2P的OER过电位为281 mV,而Ni2P和RuO2分别达到较高的过电位381 mV和325 mV. 这些结果表明微量Ce掺杂及掺杂导致的磷空位有效调节了催化剂的OER活性. Tafel斜率用来评估OER的反应动力学,一般较小的Tafel斜率意味着较快的反应动力学. Ce-Ni2P的Tafel斜率为114 mV/dec,明显低于Ni2P的131 mV/dec(图 4c),表明Ce掺杂及磷空位的形成显著促进了Ce-Ni2P的OER反应动力学. 用电化学阻抗谱进一步研究了样品在碱性溶液中发生电化学过程的电荷转移电阻. 能奎斯特图和等效电路模型如图 4e所示. 高频区域所对应的半圆与电荷转移电阻有关,半圆半径电荷转移电阻越小,催化剂导电性能越高,有利于催化过程. 拟合之后Ce-Ni2P的Rct值是1.29 Ω,明显低于纯Ni2P的Rct值4.79 Ω,说明Ce元素的加入可以进一步提高催化剂的导电性. 电化学活性表面积(ECSA)与催化活性正相关,且正比于双层电容(Cdl). 图 4d所示,Ce-Ni2P的Cdl值为1.83 mF/cm2,明显高于Ni2P的1.22 mF/cm2,表明Ce-Ni2P拥有更大的电化学活性表面积和更多的催化活性位点. 催化剂的稳定性是评估催化剂性能的另一个主要参数. 采用时间安培(I-T)测量方法研究了Ce-Ni2P在恒定电流密度为10 mA/cm2的OER过程中的电化学稳定性,如图 4f所示. 与商用RuO2相比,Ce-Ni2P在恒定电流下经198 h的连续测试,电流密度没有明显的衰减,而商用RuO2仅仅经63 h电流密度就显著衰减,表明微量Ce掺杂显著增强了催化剂的稳定性.
-
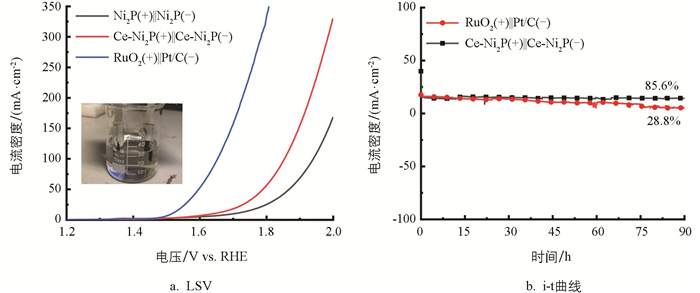
用Ce-Ni2P作为双功能材料,构建双电极电解池Ce-Ni2P(+)‖Ce-Ni2P(-),以评估催化剂的水分解活性. 碱性电解液中Ce-Ni2P(+)‖Ce-Ni2P(-),Ni2P(+)‖Ni2P(-)和商用RuO2(+)‖Pt/C(-)的水分解LSV曲线如图 5a所示. 在电流密度为10 mA/cm2时,Ce-Ni2P(+)‖Ce-Ni2P(-)的电压为1.62 V,明显低于Ni2P(+)‖Ni2P(-)的1.78 V,这意味着Ce掺杂显著改善了催化剂的水分解活性. 此外,在90 h的水分解稳定性测试后(图 5b),观测到Ce-Ni2P(+)‖Ce-Ni2P(-)电解池的电流密度略微下降,保持在85.6%,明显优于商用RuO2(+)‖Pt/C(-)电解池保持的28.8%,表明了Ce掺杂Ce-Ni2P催化剂具有良好的水分解稳定性.
2.1. 制备样品的表征
2.2. 析氧反应
2.3. 碱性水分解
-
通过水热法和煅烧法,成功制备了Ce掺杂Ni2P纳米片催化剂Ce-Ni2P. 微量Ce掺杂导致催化剂表面丰富的磷空位,并显著调控了催化剂表面的电子结构,优化了催化活性位的化学价态. 在碱性电解液中,Ce-Ni2P催化剂表现出优异的OER性能. 在50和100 mA/cm2电流密度下,Ce-Ni2P的OER过电位分别为241和281 mV,显著优于Ni2P和商用RuO2的过电位. 此外,该催化剂展现出长期的OER稳定性.






 DownLoad:
DownLoad: