-
开放科学(资源服务)标识码(OSID):

-
湿热泄泻(Damp-heat Diarrhea,DHD)又称泄泻·湿热蕴结证,指机体在外源致病因子、饮食不节/洁等因素影响下湿热之邪蕴结于肠内,以热证、湿证为主的泄泻证候.相关研究显示,DHD大鼠回肠杯状细胞的密度极显著降低、潘氏细胞的密度极显著升高[1];DHD仔猪回肠中大肠杆菌数量极显著上升,乳酸杆菌数量显著降低,乳酸杆菌与大肠杆菌比值极显著下降,且回肠IL-2 mRNA表达量显著上升,IL-4,IL-5 mRNA表达量显著降低[2];DHD大鼠回肠绒毛上皮脱落、黏膜固有层出血[3];DHD仔猪回肠Notch-1,Hes-1 mRNA的表达量显著升高、Hath-1 mRNA的表达量显著降低[4].
白头翁汤(Pulsatilla Decoction,PD)出自《伤寒杂病论》,是由白头翁、黄连、黄柏、秦皮组成的传统中药方剂,具有清热解毒、凉血止痢的功效,为治疗热毒痢疾的原始处方.方中以白头翁为君,清热解毒,凉血止痢;臣以黄连之苦寒,清热解毒,燥湿厚肠;黄柏泻下焦湿热,共奏燥湿止痢之效;秦皮苦寒性涩,收敛作用强,用以止血.四药并用,为热毒血痢之良方,临床上常用于治疗阿米巴痢疾、细菌性痢疾等病证.近年来,PD治疗肠道病证的相关研究发现,PD通过显著降低血清中IL-2,IFN-γ,DAO,HIF-1α及CD8+水平且显著提高IL-4,IL-10,CD4+以及CD4+/CD8+水平,来调节Th1/Th2细胞平衡和激活免疫机制[5];PD可显著降低小鼠血清中IL-6和TNF-α水平,显著降低结肠组织中p-mTOR/mTOR,p-P70S6K/P70S6K,p-STAT3/STAT3,COX-2 mRNA的表达量,从而预防小鼠溃疡性结肠炎[6].PD可抑制TNF-α mRNA的表达量从而抑制溃疡性结肠炎的炎症反应,促进肠黏膜愈合[7];PD可抑制TLR4/NF-κB信号通路,下调P-选择素,MPO,MIF,TXB2的水平,促进肠道黏膜的修复,减轻溃疡性结肠炎大鼠结肠炎症反应[8-9].
本课题组前期研究发现,PD对DHD有良好的治疗效果,可以缓解DHD肠道组织损伤[9].为进一步探究PD对DHD大鼠回肠黏膜屏障功能及色氨酸代谢通路的影响,本试验建立DHD大鼠模型,并以不同剂量PD进行灌胃治疗,分析大鼠回肠组织形态变化、回肠肠道黏膜屏障损伤、肠道炎症反应及色氨酸代谢通路之间的关联,以期为探索PD介导改善DHD的潜在机制提供新见解.
HTML
-
48只SPF级雄性SD大鼠,7周龄,体质量180~200 g,购自湖南斯莱克景达实验动物有限公司[SCXK(湘)2019-0004],饲养于西南大学动物医学院实验室[SYXK(渝)2019-0003],造模前适应性饲养7 d,自由采食及饮水,环境温度为20~24 ℃.所有操作均符合西南大学动物实验伦理学要求(审批号:IACUC-20210507-06).
-
大肠杆菌(南京农业大学分离鉴定的HE株[10])由南京农业大学馈赠;蜂蜜(GB14963):江西华茂保健品开发有限公司;猪油:重庆市荣昌区水口寺菜市场;56度红星二锅头(GB/T10781.2):北京红星股份有限公司;苏木素伊红染色液(20220610):湖南比克曼生物科技有限公司;Rat IL-1β ELISA KIT(YJ003075)及Rat IL-6 ELISA KIT(YJ064292):上海源桔生物科技中心;TRIzol试剂:Beyotime,上海;PrimeScriptTM RT Master Mix(RR036A)及TB Green © Premix Ex TaqTM Ⅱ(RR820A):宝日医生物技术(北京)有限公司;兽用全自动血液细胞分析仪(BC-2600Vet):深圳迈瑞生物医疗电子股份有限公司;轮式切片机(KD1508A):浙江金华科迪仪器设备有限公司;全自动酶标仪(A-5082):Tecan Austria GmbH;实时荧光定量PCR仪(Archimed X6):杭州鲲鹏基因科技有限责任公司.
-
白头翁、秦皮、黄连、黄柏购置于西城大药房(中国重庆荣昌)药材公司,经过西南大学动物医学院曹立亭副教授鉴定,药材信息如表 1所示.根据2020年版《中华人民共和国兽药典》关于白头翁散的配比称取白头翁60 g、秦皮45 g、黄连30 g、黄柏60 g,加10倍水浸泡30 min,大火煮沸后转小火煎煮40 min,过滤收集滤液后再加8倍量水继续煎煮,如此重复2次,合并3次滤液,浓缩至0.188 g/mL作为PD低浓度、0.376 g/mL作为PD中浓度、0.752 g/mL作为PD高浓度.
-
试验前1 d,称量每只SD大鼠体质量,用分层随机法将48只大鼠随机分为空白组(NC)、模型组(Model)、自愈组(SH)、白头翁汤高剂量组(PD-H)、白头翁汤中剂量组(PD-M)、白头翁汤低剂量组(PD-L),每组8只大鼠,平均体质量为(254±3)g.除NC外,分3个阶段建立DHD模型,分别为高糖高脂、高热高湿及攻毒阶段,全程给予30%蜂蜜水.高糖高脂阶段为1~10 d,第1,3,5,7,9 d给予每只大鼠4 mL猪油,自由采食和饮用蜂蜜水,第2,4,6,8,10 d禁食不禁水;高热高湿阶段为11~15 d,此阶段给予每只大鼠2 mL红星二锅头,自由采食饮水,每日需在自建高温湿度棚[温度(34±1) ℃,湿度(94±1) %]放置8 h;攻毒阶段为16~18 d,Model、SH、PD-H、PD-M、PD-L大鼠于第16 d及17 d注射浓度为3.96×1011 CFU/mL大肠杆菌0.2 mL,此阶段均自由采食及饮水.NC饲喂方式与适应性饲养方法相同.治疗阶段为19~23 d,造模成功后,PD-H、PD-M、PD-L大鼠分别灌胃不同剂量PD并于第19 d处死Model组大鼠.于造模前后每日定时记录各组大鼠体质量、采食量,各组大鼠实验处理情况如表 2所示.
-
试验结束后,称各组大鼠体质量并将各组大鼠处死及时称质量和记录大鼠心、肝、脾、肺、肾的质量,根据各脏器质量/体质量得到大鼠各脏器指数.采集各组大鼠回肠组织取适宜长度固定于4%中性甲醛溶液,另取一部分回肠组织保存于-80 ℃冰箱备用.
-
采集各组大鼠20 μL尾尖血,加入稀释液轻轻吹打混匀,于西南大学动物医院对大鼠血常规指标进行检测.腹主动脉采集血液并分离血浆,按照ELISA试剂盒说明书要求测定大鼠血浆IL-6,IL-1β.
-
取固定的各组大鼠回肠组织,冲水后经75%~100%乙醇梯度脱水并透明,浸蜡1.5 h,重复2次.包埋后切片、展片,烘片后使用二甲苯浸泡脱蜡,重复2次再经100%~75%乙醇梯度复水,苏木素伊红染色后75%~100%乙醇梯度脱水,二甲苯透明后即可用中性树胶封片以备回肠组织形态学观察,并测定回肠绒毛高度和隐窝深度,计算绒毛高度和隐窝深度的比值.
-
取-80 ℃储存的各组大鼠回肠组织用TRIzol试剂提取总RNA,利用PrimeScriptTM RT Master Mix试剂盒说明书(含gDNase)反转录获得cDNA,存于-20 ℃备用.使用QuantStudioTM 7 Flex实时荧光定量PCR系统和SuperReal PreMix Plus(SYBR Green)进行qPCR. RT-qPCR反应体系为TB Green ©Premix Ex TaqTMⅡ(2×)5 μL,上、下游引物各0.4 μL,ROX Reference Dye (50×)0.2 μL,无酶无菌水3 μL,DNA模板1 μL.以GAPDH作为内参基因.反应条件为95 ℃预变性30 s;95 ℃ 5 s变性,60 ℃ 34 s退火,反应40个循环60 ℃ 1 min延伸,95 ℃ 15 s熔解.数据分析采用2-ΔΔCt方法.使用的引物序列如表 3所示.
-
结果以平均值±标准差表示,用IBM SPSS 26.0软件分析试验数据,并使用单因素方差分析(ANOVA)组间差异,然后进行LSD事后检验,p<0.05表示差异有统计学意义.用Prism Grappad 9.0作图.
1.1. 材料
1.1.1. 试验动物
1.1.2. 主要试剂与仪器
1.2. 方法
1.2.1. 白头翁汤的制备
1.2.2. 动物分组及处理
1.2.3. 样品采集
1.2.4. 血液指标检测
1.2.5. 回肠组织形态学观察
1.2.6. 回肠炎症反应、色氨酸代谢及肠道黏膜屏障mRNA表达水平测定
1.2.7. 统计学分析
-
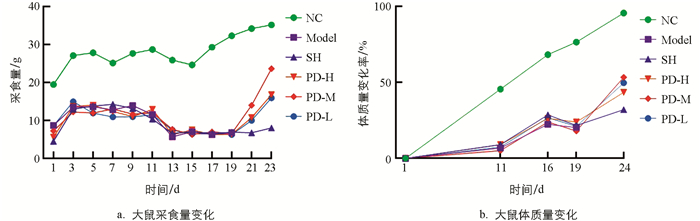
如图 1a所示,试验中高糖高脂、高热高湿、攻毒及治疗阶段大鼠采食量起伏波动:高糖高脂阶段大鼠采食量初上升后平稳,与NC组相比,Model组大鼠采食量明显降低,SH组、PD-H组、PD-M组、PD-L组大鼠与Model组相比,差异无统计学意义;高热高湿阶段大鼠采食量先急剧下降后逐渐平稳,与NC组相比,Model组大鼠采食量明显下降,SH组、PD-H组、PD-M组、PD-L组与Model组相比,差异无统计学意义;攻毒阶段,与NC组相比,Model组大鼠采食量明显降低,SH组、PD-H组、PD-M组、PD-L组与Model组相比,差异无统计学意义;治疗阶段,与NC组相比,SH组大鼠采食量明显降低,与SH组相比,PD-H组、PD-M组、PD-L组大鼠采食量明显上升,且PD-M组大鼠采食量高于PD-H组和PD-L组.
如图 1b所示,试验中高糖高脂、高热高湿、攻毒及治疗阶段大鼠体质量增长率各不相同:Model组、SH组、PD-H组、PD-M组和PD-L组大鼠高糖高脂阶段体质量变化率分别为6.7%,8.9%,9.0%,5.0%,7.1%,低于NC组的45.6%;高热高湿阶段Model组、SH组、PD-H组、PD-M组、PD-L组大鼠体质量变化率分别为22.2%,28.6%,26.2%,23.8%,26.4%,低于NC组的68.4%;攻毒阶段Model组、SH组、PD-H组、PD-M组、PD-L组大鼠体质量变化率分别为20.0%,21.5%,23.8%,17.8%,20.8%,均低于NC组的76.8%;治疗阶段,PD-H组、PD-M组、PD-L组大鼠体质量变化率均高于SH组大鼠,且4组均低于NC组.
-
如表 4所示,造模成功后,Model组大鼠的淋巴细胞数(Lymph)、单核细胞数(Mon)、中性粒细胞绝对值(Gran)、淋巴细胞百分比(Lymph%)、红细胞数(RBC)、平均红细胞体积(MCV)、血小板数(PLT)都发生了显著变化:与NC组相比,Model组大鼠Lymph,Mon,MCV显著降低(p<0.05),Gran,Lymph%,RBC,PLT显著升高(p<0.05),而SH组大鼠白细胞总数(WBC)、血红蛋白数(HGB)均显著降低(p<0.05),即WBC,HGB显著改变发生于DHD大鼠自愈恢复期.与Model相比,PD-H组大鼠Lymph,MCV显著升高(p<0.05);PD-M组大鼠Lymph,Mon,PLT均显著降低(p<0.05),而HGB,MCV显著升高(p<0.05);PD-L组大鼠HGB显著降低、MCV显著升高(p<0.05).
-
如表 5所示,造模后,Model组大鼠的心脏、肝脏、脾脏、肺脏、肾脏指数与NC组比较均显著上升(p<0.05).与Model组相比,PD-H组、PD-M组、PD-L组大鼠情况各异:PD-H组、PD-L组大鼠心脏指数有降低趋势,但差异无统计学意义;PD-M组大鼠肝脏指数显著降低(p<0.05),PD-H组、PD-L组大鼠有所降低,但差异无统计学意义,PD-H组、PD-M组、PD-L组大鼠脾脏指数均显著降低(p<0.05),PD-H组、PD-L组大鼠肺脏、肾脏指数显著降低(p<0.05),PD-M组大鼠有降低趋势,但差异无统计学意义;SH组大鼠肝脏、脾脏、肺脏指数均显著降低(p<0.05).
-
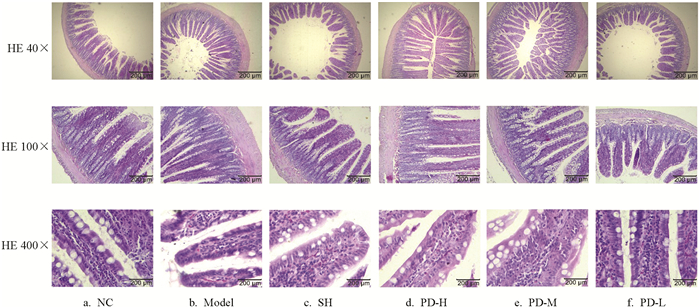
图 2a显示,NC组大鼠回肠在低倍镜下形态结构完整,肠绒毛致密,肠绒毛排列整齐无断裂,固有层连接紧密无增厚;高倍镜下绒毛轮廓清晰,隐窝结构清晰完整,微绒毛形成的纹状缘清晰完整,杯状细胞排列整齐,乳糜管结构清晰无异常.图 2b显示,Model组大鼠回肠低倍镜下结构完整,肠绒毛过于密集,肠绒毛排列较杂乱,固有层明显增厚;高倍镜下隐窝结构有消失,杯状细胞数量减少,微绒毛形成的纹状缘弥漫性缺失,乳糜管内有红细胞浸润,说明有出血.图 2c显示,SH组大鼠回肠低倍镜下结构紊乱,肠绒毛形状各异、排列紊乱,固有层厚薄不一;高倍镜下隐窝结构有消失,杯状细胞排列紊乱,纹状缘弥漫性缺失,乳糜管增宽内有红细胞浸润及炎性细胞,说明有出血和炎症.图 2d显示,PD-H组大鼠回肠低倍镜下结构完整,肠绒毛形状完整、排列整齐,固有层厚薄不一;高倍镜下隐窝结构明显,杯状细胞有增多、排列整齐,乳糜管增宽内有红细胞浸润,说明有出血.图 2e显示,PD-M组大鼠回肠低倍镜下结构完整,肠绒毛形状各异、排列紧密而紊乱,固有层厚薄不一;高倍镜下隐窝有消失,杯状细胞有增多、排列紊乱,乳糜管增宽内有红细胞浸润,说明有出血.图 2f显示,PD-L组大鼠回肠低倍镜下结构完整,肠绒毛形状各异、排列疏松而紊乱,固有层变薄;高倍镜下隐窝变短,杯状细胞排列紊乱,乳糜管增宽内有红细胞浸润,说明有出血.
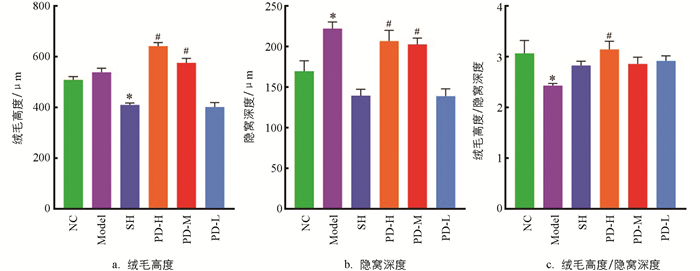
测定各组大鼠回肠绒毛高度、隐窝深度,计算绒毛高度与隐窝深度的比值[11],结果如图 3a所示.与NC组比较,Model组的变化并不显著,但SH组大鼠回肠绒毛高度显著降低(p<0.05);与SH组比较,PD-H组、PD-M组回肠绒毛高度显著升高(p<0.05).如图 3b所示,与NC组比较,Model组大鼠回肠隐窝深度显著升高(p<0.05);与Model组比较,PD-H组、PD-M组回肠隐窝深度显著降低(p<0.05).如图 3c所示,与NC组比较,Model组大鼠回肠绒毛高度/隐窝深度的值显著降低(p<0.05);与Model组比较,PD-H组大鼠回肠绒毛高度/隐窝深度的值显著升高(p<0.05).
-
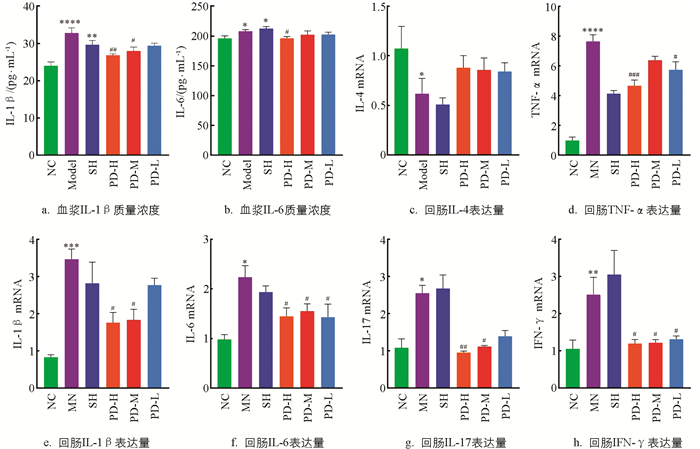
如图 4a,e所示,与NC组比较,Model组大鼠血浆IL-1β质量浓度与回肠组织IL-1β mRNA表达量极显著升高(p<0.01);与Model组比较,PD-H组、PD-M组大鼠显著降低(p<0.05),PD-L组大鼠降低但不显著;SH组大鼠IL-1β质量浓度、mRNA表达量均未恢复.如图 4b,f所示,与NC组比较,Model组大鼠血浆IL-6质量浓度与回肠组织IL-6 mRNA表达量均显著升高(p<0.05);与Model组比较,PD-H组大鼠血浆IL-6质量浓度显著降低(p<0.05),PD-H组、PD-M组、PD-L组大鼠回肠组织IL-6 mRNA表达量均显著降低(p<0.05).
如图 4c所示,与NC组比较,Model组大鼠回肠组织IL-4 mRNA表达量显著降低(p<0.05);与Model组比较,PD-H组、PD-M组、PD-L组大鼠回肠IL-4 mRNA表达量无显著差异.如图 4g所示,与NC组比较,Model组大鼠回肠IL-17 mRNA表达量显著升高(p<0.05);与Model组比较,PD-H组大鼠回肠IL-17 mRNA表达量极显著降低(p<0.01)、PD-M组显著降低(p<0.05).如图 4d所示,与NC组比较,Model组大鼠回肠组织TNF-α mRNA表达量显著升高(p<0.05);与Model组比较,PD-H组、PD-L组大鼠回肠组织TNF-α mRNA表达量显著降低(p<0.05).如图 4h所示,与NC组比较,Model组大鼠回肠组织IFN-γ mRNA表达量显著升高(p<0.05);与Model组比较,PD-H组、PD-M组、PD-L组大鼠回肠组织IFN-γ mRNA表达量显著降低(p<0.05).
-
如图 5所示,与NC组比较,除KYNU外,Model组大鼠回肠组织TPH1,KMO,IDO-1 mRNA表达量均显著升高(p<0.05).与Model组比较,PD-H组、PD-M组、PD-L组大鼠回肠组织TPH1,KMO mRNA水平均显著降低(p<0.05),PD-H组的KYNU mRNA水平显著降低,PD-H组、PD-M组大鼠回肠组织IDO-1 mRNA水平显著降低(p<0.05).
-
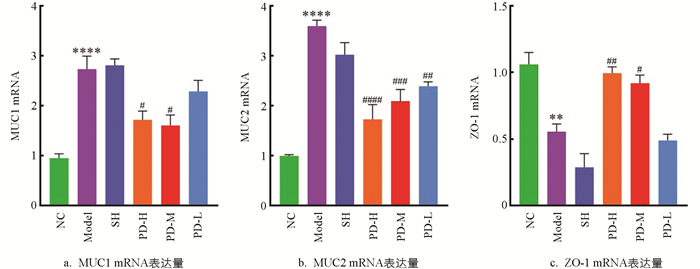
如图 6所示,与NC组比较,Model组大鼠回肠组织的MUC1,MUC2 mRNA表达水平显著升高(p<0.05),ZO-1 mRNA表达水平显著降低(p<0.05).与Model组比较,PD-H组、PD-M组、PD-L组治疗后大鼠的MUC1,MUC2 mRNA水平均降低,其中,PD-H组、PD-M组显著降低MUC1的mRNA表达水平(p<0.05);PD-H组、PD-M组、PD-L组极显著降低MUC2的mRNA表达水平(p<0.01);PD-H组极显著升高ZO-1的mRNA表达水平(p<0.01),PD-M组显著升高ZO-1的mRNA表达水平(p<0.05).
2.1. PD对DHD大鼠体质量和采食量的影响
2.2. PD对DHD大鼠血常规指标的影响
2.3. PD对DHD大鼠脏器指数的影响
2.4. PD对DHD大鼠回肠组织形态的影响
2.5. PD对DHD大鼠炎症细胞因子血浆质量浓度及mRNA表达量的影响
2.6. PD对DHD大鼠回肠色氨酸代谢相关基因表达量的影响
2.7. PD对DHD大鼠回肠肠道黏膜屏障相关基因mRNA表达量的影响
-
本试验各组大鼠血常规指标的差异说明DHD大鼠处于炎症阶段,Model组、SH组大鼠Lymph,Mon显著降低,而经不同剂量的PD治疗后大鼠的Lymph,Mon显著回升或有所回升,说明大鼠处于DHD阶段机体血液中淋巴细胞、单核细胞显著降低,这无疑增加机体继发感染的风险,降低了机体的免疫力,而不同剂量的PD均不同程度地阻止了淋巴细胞、单核细胞数目的减少,有利于维持机体的抗感染环境,并能有效阻止炎性细胞减少.Model组大鼠Gran,Lymph%显著上升而经PD治疗后有所减少,说明DHD大鼠机体内有大量致病因子,Gran也被称为“小吞噬细胞”,一方面Gran的增多有利于吞噬病原、衰老损伤的细胞及细胞因子,有利于增强细胞免疫反应[12],但另一方面Gran过多也不利于机体炎性稳态,可能会损伤其他健康细胞.Model组大鼠RBC显著上升、MCV显著降低,结合DHD大鼠出现的腹泻、血便等临床症状说明DHD大鼠腹泻情况较为严重,机体存在脱水现象,而经PD治疗后RBC回调或显著阻止MCV降低,说明PD可缓解DHD大鼠的腹泻脱水症状.Model组大鼠PLT显著升高,结合回肠组织病理切片结果可得DHD大鼠回肠处有出血,需血小板发挥止血作用,因此,Model组大鼠血小板数量急剧上升,PD可显著缓解DHD大鼠的出血症状,阻止血小板的急剧增多,这有利于阻止血栓的形成从而保护DHD大鼠回肠.
DHD大鼠的IL-1β,IL-6 mRNA表达量显著升高,而经PD治疗后DHD大鼠回肠组织IL-1β,IL-6 mRNA表达量显著降低.Kaminsky等[13]研究发现IL-1β通过丝裂原活化蛋白激酶(MAPKs)信号转导途径和其他转录因子诱导增加肠道通透性.IL-6与其他炎症细胞因子相互作用,引发炎症反应[14],Guo等[15]研究发现IL-6介导的Jak/STAT3途径可能通过其下游PI3激酶/Akt信号肽驱动杯状细胞分化,IL-6是损伤后组织修复所必需的,能促进隐窝中潘氏细胞的增殖从而诱导肠上皮增殖,IL-6有助于通过经典信号传导和反式信号通路维持肠道稳态.本研究中,DHD大鼠回肠组织IL-1β,IL-6的mRNA表达量显著升高,可能会增加回肠通透性,易引起炎性风暴,会增加对机体的损伤[16].PD治疗后,DHD大鼠血浆IL-1β,IL-6质量浓度和回肠组织mRNA表达量回调,表明PD可能通过保护回肠的通透性、降低炎症、促进回肠上皮增生来保护回肠,减轻回肠损伤.
本研究中,DHD大鼠的IL-4表达量显著降低,IL-17,IFN-γ,TNF-α mRNA表达量显著升高.研究表明,回肠组织IL-4 mRNA的表达量可反映出回肠黏膜炎症的变化趋势,IL-4不仅能促进淋巴细胞分化、使其增殖能力增强,还可促进体液免疫增强,刺激B细胞、嗜酸性粒细胞、嗜碱性粒细胞、肥大细胞的活化[17],而且IL-4具有一定的免疫抑制作用.Lee等[18]研究发现,IL-17缺乏会降低DSS诱导的肠黏膜损伤期间维持屏障功能的能力,IFN-γ,TNF-α影响上皮通透性[19],增加肠道通透性,从而进一步暴露于管腔内容物,并引发免疫反应和肠道炎症[20],经PD治疗后,上述肠道炎症反应相关基因不同程度回调,表明PD有利于阻止DHD大鼠回肠上皮通透性的增加,维持回肠黏膜屏障功能.
色氨酸代谢在炎症性肠炎中特别容易受到干扰[21],KYNU(犬尿素酶)、TPH1(色氨酸羟化酶1)、KMO(犬尿氨酸3-单加氧酶)、IDO-1(吲哚胺2,3-双加氧酶)是色氨酸代谢相关通路的关键因子.Model组大鼠回肠组织TPH-1,KYNU,KMO,IDO-1的mRNA水平显著升高,表明DHD大鼠色氨酸代谢的2条途径即5羟色胺途径与犬尿氨酸都显著加强,色氨酸代谢通路被过度激活,经不同剂量的PD治疗后TPH-1,KYNU,KMO,IDO-1的mRNA水平极显著或显著降低,表明PD可调节回肠组织色氨酸代谢.
MUC1,MUC2,ZO-1为紧密连接蛋白,是回肠黏膜屏障的重要组成部分[22],黏蛋白构成黏液层内层,由上皮细胞分泌,是凝胶状分泌物的关键成分,具有调节组织黏膜屏障、参与细胞信号传导以介导机体免疫等生物学功能[23],紧密连接蛋白ZO-1是肠黏膜免疫的关键蛋白,其基因表达量常被用于肠机械屏障功能的关键性检测标识[24]. 结合回肠组织形态学观察结果,Model组、SH组大鼠的回肠组织病理损伤严重,MUC1,MUC2的mRNA表达量急剧上升,ZO-1 mRNA的表达量显著下降,PD可显著降低肠黏膜损伤,PD组大鼠的MUC1,MUC2的mRNA表达量显著降低或降低,ZO-1 mRNA表达量显著上升.
-
本研究以“高糖高脂+高热高湿+肠毒性大肠杆菌”为诱导条件建立DHD大鼠模型,并以不同剂量的PD进行灌胃治疗.结果表明,经PD灌胃治疗后,大鼠回肠形态结构有所恢复,隐窝病变缓解.PD可以减轻大鼠IL-1β,IL-6,IL-17,TNFα,IFN-γ等促炎因子的生成,促进抑炎因子IL-4的生成来调节回肠炎症反应.PD可以降低TPH-1,KYNU,KMO,IDO-1的表达量从而阻止色氨酸代谢通路过度激活以调节色氨酸代谢,有效缓解DHD引起的肠道代谢紊乱.同时,PD阻止MUC1,MUC2升高、ZO-1降低从而使大鼠回肠黏膜屏障趋于稳态.







 DownLoad:
DownLoad: