-
开放科学(资源服务)标识码(OSID):

-
自噬最早于1963年由De Duve等首次提出,用来描述细胞自身物质的溶酶体降解途径[1]。随后,Tsukada等在酵母上鉴定出第一个自噬基因APG[2],之后陆续在酵母、动物和植物中鉴定出40多个自噬相关蛋白(ATGs)。早期对自噬的研究主要集中于其在癌症、神经退行性疾病等人类疾病中的功能[3]。Liu等[4]首次发现烟草花叶病毒(Tobacco mosaic virus,TMV)的侵染能够诱导自噬基因的表达。随后,越来越多的研究表明,自噬反应在植物应对多种植物病毒感染时被激活,不仅能够限制病毒的积累,还能促进病毒的感染,是植物响应病毒侵染的重要调控机制[5-6]。在发生自噬反应的植物细胞中可以观察到自噬相关基因(ATGs)表达量显著上调,以及自噬小体和自噬标记物ATG8的大量积累[7]。
双生病毒(Geminiviruses)是一类在世界范围内广泛发生的单链环状DNA病毒,其寄主范围广泛,主要通过烟粉虱、叶蝉和蚜虫传播,每年造成的经济损失达数百亿美元。自噬现象在双生病毒上也较为普遍,棉花木尔坦曲叶病毒(Cotton leaf curl Multan virus,CLCuMuV)编码的βC1蛋白是第一个被鉴定的植物病毒自噬激活剂,通过阻止自噬相关蛋白3(ATG3)与胞质自噬负调控因子3-磷酸甘油醛脱氢酶(GAPC)互作,从而激活植株的自噬反应,抑制CLCuMuV侵染[8]。随后发现云南番茄曲叶病毒(Tomato leaf curl Yunnan virus,TLCYnV)、印度绿豆黄化花叶病毒(Mungbean yellow mosaic India virus,MYMIV)、甜菜严重曲顶病毒(Beet severe curly top virus,BSCTV)等多种双生病毒都能激活细胞自噬途径,上调自噬相关基因mRNA的表达[9-10]。
柑橘褪绿矮缩病毒(Citrus chlorotic dwarf-associated virus,CCDaV)是近年来柑橘生产上出现的一种新病毒,自19世纪80年代在土耳其东部以及地中海地区默辛省被首次发现[11],目前在中国和泰国都有发生[12-13]。CCDaV在柠檬、红宝石柚等敏感品种上引起叶片褪绿、斑驳和扭曲(图 1a和1b)。CCDaV基因组为约3.64 kb的环状单链DNA分子,可能含有6个开放阅读框(Open reading frames,ORFs),分别是V1、V2、V3、V4、RepA、Rep。其中,V1被认为是外壳蛋白(CP);V2含有双生病毒V2蛋白的保守结构域,具有保守的半胱氨酸残基,该残基对抑制番茄黄化曲叶病毒(Tomato yellow leaf curl virus,TYLCV)诱导的基因沉默至关重要[14]。近来研究发现WRKY1正向调控CCDaV复制相关蛋白RepA在本生烟(Nicotiana benthamiana)中诱导细胞死亡[15]。CCDaV编码的V2蛋白可抑制GFP RNA引发的局部和系统RNA沉默,但不影响16c本生烟中RNA沉默信号的短距离移动[16]。目前国内外尚未开展关于CCDaV诱导寄主自噬的相关研究。
本研究以感染CCDaV的尤力克柠檬作为研究材料,探究CCDaV是否能诱导尤力克柠檬的自噬反应,以及诱导自噬的病毒蛋白种类,以期为今后研究细胞自噬在CCDaV侵染寄主中的调控作用提供参考。
HTML
-
无病毒一年生尤力克柠檬实生苗和CCDaV毒株均保存于国家柑桔苗木脱毒中心。本生烟生长条件为25 ℃,16 h光照,8 h黑暗处理。统一采用生长至40 d的四至六叶期烟苗为试验材料。
-
植物蛋白提取试剂盒购自北京索莱宝生物科技有限公司;IsoPlus、Prime STAR Max DNA Polymerase购自宝生物工程(大连)有限公司;氯仿、乙醇、异丙醇等购自北京鼎国昌盛生物技术有限责任公司;反转录一步法试剂购自ABM公司;实时荧光定量SYBR Prime qPCR Mix试剂购自重庆葆光生物科技有限公司;Anti-Actin抗体、羊抗鼠二抗和羊抗兔二抗购买于翌圣生物科技(上海)股份有限公司;GFP单克隆抗体、ATG8单克隆抗体购自成都AlpVHHs有限公司。
-
使用直径5 mm打孔器在感染CCDaV 15 d后的尤力克柠檬嫩叶上取样,使用透射电镜观察自噬结构。统计超过50个细胞内自噬小体的平均值,进行3次重复试验。使用GraphPad Prism 8软件进行统计学分析。
-
称取0.1 g感染CCDaV 15 d后的尤力克柠檬嫩叶,IsoPlus提取总RNA反转录为cDNA。使用SYBR Prime qPCR Mix进行qRT-PCR反应。以尤力克柠檬的ClActin作为内参基因。采用2-ΔΔct法分析相对表达量,GraphPad Prism 8软件进行t检验分析。
-
利用Prime STAR Max DNA Polymerase,以CCDaV毒株为模板扩增CCDaV的V1、V2、V3、V4、C1和C2基因,并将其分别构建到pART27-Myc中。测序验证后,分别与GFP-NbATG8f转化农杆菌GV3101,并共注射于本生烟;48 h后使用Olympus FV3000进行观察;488 nm观察绿色荧光(GFP),543 nm观察红色叶绿体自发光;浸润pART27-Myc-GUS的本生烟作为对照。
-
称取0.2 g感染CCDaV 15 d后的尤力克柠檬嫩叶,使用植物组织蛋白提取试剂盒提取总蛋白,并进行12.5% SDS-PAGE。分别使用CCDaV CP单克隆抗体和ATG8单克隆抗体作为一抗室温孵育2 h,再分别以羊抗鼠二抗和羊抗兔二抗室温孵育2 h,Myc直标抗体室温孵育2 h。使用IMAGEJ软件分析蛋白条带灰度值。
1.1. 供试材料
1.2. 主要试剂
1.3. 透射电子显微镜检测自噬
1.4. 实时荧光定量检测
1.5. 诱导自噬的病毒蛋白种类鉴定
1.6. Western Blot检测自噬
-
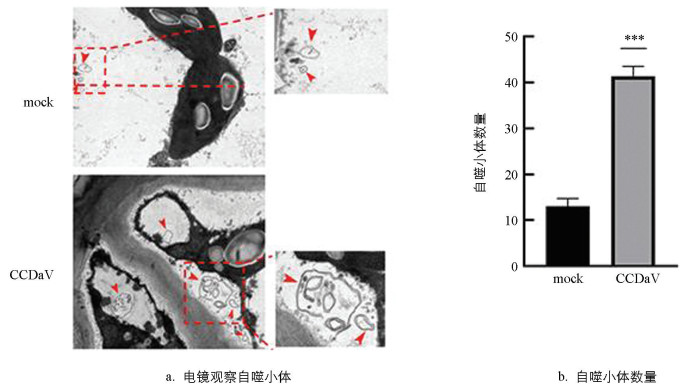
CCDaV侵染尤力克柠檬15 d后,在透射电子显微镜下观察到有双层膜囊泡状结构的自噬小体(图 2a)。经统计分析,感病尤力克柠檬叶片中自噬结构数量显著高于无病毒尤力克柠檬(图 2b)。结果表明,CCDaV可能诱导尤力克柠檬叶片细胞内的自噬反应。
-
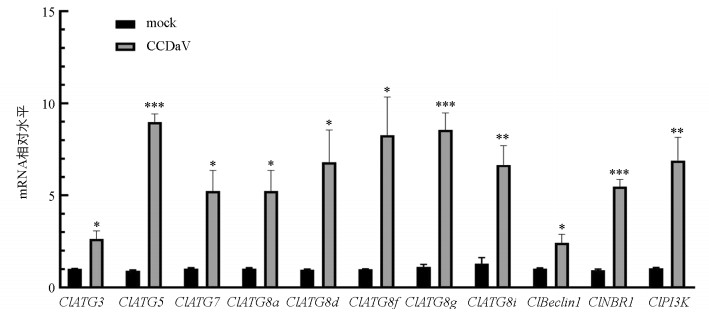
进一步qRT-PCR检测尤力克柠檬嫩叶中自噬相关基因的表达量发现,感染CCDaV 15 d后,自噬相关基因ClBeclin1、ClPI3K、ClNBR1、ClATG3、ClATG5、ClATG7、ClATG8a、ClATG8d、ClATG8f、ClATG8i和ClATG8g的表达量与对照组相比均显著上调。其中ClATG5、ClATG8g、ClNBR1、ClATG8i、ClPI3K的mRNA表达水平增长最明显,为对照组的6.64~10.52倍,另外ClBeclin1、ClATG3、ClATG7、ClATG8a、ClATG8d、ClATG8f的mRNA表达水平为对照组的2.64~5.24倍(图 3)。结果表明CCDaV侵染尤力克柠檬激活了自噬相关基因的表达。
-
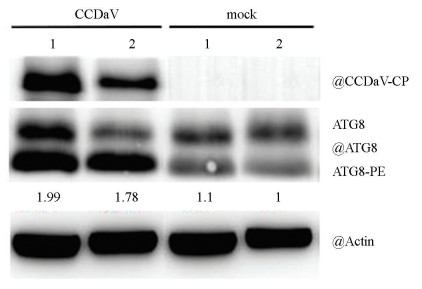
尤力克柠檬接种CCDaV 15 d后,蛋白印迹检测发现ATG8和ATG8-PE的积累量均显著增加(图 4)。与对照组相比,感病叶片细胞内ATG8-PE的积累增加了1.99倍。结果进一步表明CCDaV侵染激活了尤力克柠檬中的细胞自噬反应。
-
通过PCR扩增分别得到CCDaV编码的6个ORFs全长序列:C1(810 bp)、C2(243 bp)、V1(765 bp)、V2(420 bp)、V3(300 bp)和V4(251 bp)。
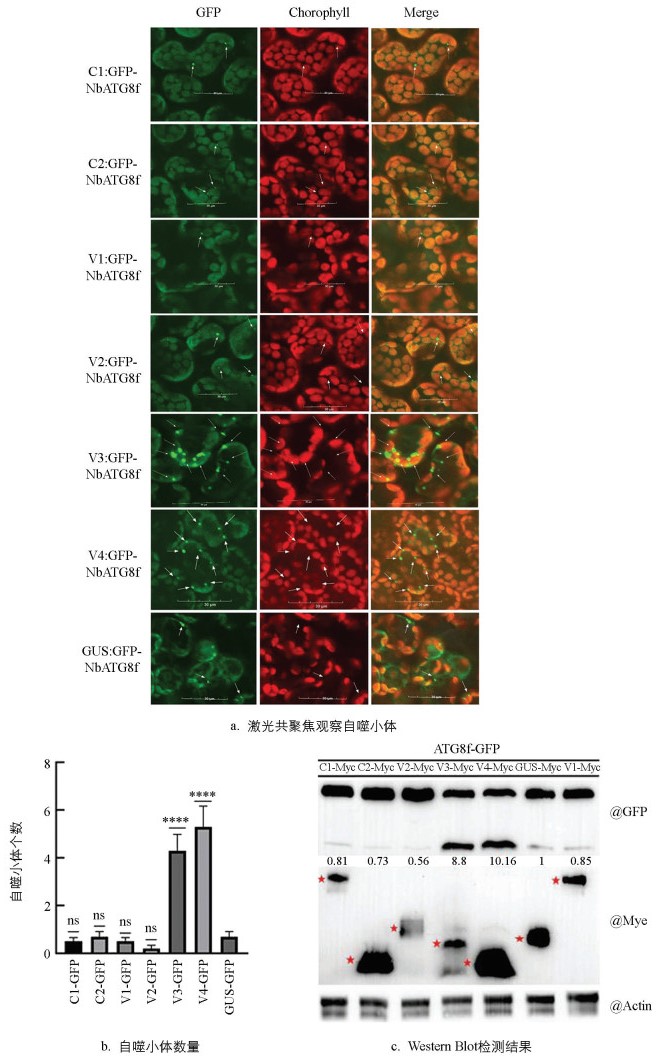
将CCDaV编码的各个蛋白分别与GFP-NbATG8f共表于本生烟48 h后,通过共聚焦观察发现,V3和V4蛋白,分别与GFP-NbATG8f共表达时,自噬小体数量迅速增加。CCDaV编码的其他蛋白分别与GFP-NbATG8f共表达时,本生烟中的自噬小体数量与对照(pART27-Myc-GUS)相似(图 5a、5b)。蛋白印迹分析的结果进一步显示,与对照组相比,V3和V4分别与GFP-NbATG8f共表达时,游离GFP的积累量分别增加了8.80倍和10.16倍(图 5c)。上述结果表明,V3、V4蛋白可能是CCDaV诱导自噬的重要因子。
2.1. 透射电镜观察细胞自噬
2.2. CCDaV侵染激活自噬相关基因的表达
2.3. CCDaV侵染诱导自噬体标志蛋白ATG8-PE表达
2.4. CCDaV编码蛋白诱导细胞自噬能力鉴定
-
自噬是一种真核生物中的重要保护过程,当植物受到特定信号刺激,如营养匮乏、温度胁迫、渗透胁迫或病原物侵染时,自噬会被大量诱导以清除受损细胞器或异常蛋白[17-18]。前期研究显示,柑橘黄化脉明病毒(Citrus yellow vein clearing virus,CYVCV)编码的病毒蛋白TGB2、TGB3和CP分别与GFP-ATG8f共表于本生烟48 h后自噬相关基因表达显著上调[19]。本研究发现,与对照相比,CCDaV侵染尤力克柠檬15 d后,在叶肉细胞中出现了明显的双层膜囊泡结构,液泡中也出现了类似自噬小体的结构。此时,ClATG3、ClATG5、ClATG7、ClATG8a、ClATG8d、ClATG8f、ClATG8g、ClATG8i、ClBeclin1、ClPI3K和ClNBR1等自噬相关基因的表达量显著上调。由此证明CCDaV可诱导植株发生细胞自噬反应。
自噬在植物响应病原菌侵染中发挥了重要作用。例如,自噬相关蛋白ATG2负调控拟南芥对白粉病的抗性和白粉菌诱导的细胞死亡[20]。灰霉菌(Botrytis cinerea)诱导拟南芥自噬基因ATG18a的表达和自噬体的形成[21]。柑橘黄龙病菌(Candidatus liberibacter asiaticus)分泌蛋白SDE3抑制寄主自噬促进黄龙病菌侵染[22]。此外,自噬在植物抵御病毒侵染中也发挥了重要的作用。Jiang等[23]研究发现水稻条纹病毒(Rice stripe virus,RSV)的P3蛋白可以与本生烟中的NbPI3P相互作用,从而抑制P3作为沉默抑制子的活性,并诱导P3被自噬降解,进而提高植株对RSV的抗性。此外,烟草钙调蛋白样蛋白rgs-Ca可通过与烟草蚀纹病毒(Tobacco etch virus,TEV)编码的HC-Pro,以及黄瓜花叶病毒(Cucumber mosaic virus,CMV)和番茄不孕病毒(Tomato aspermy virus,TAV)编码的2b蛋白结合,从而诱导自噬,限制病毒侵染。为成功侵染植物,病毒也进化出多种手段来抑制植物的自噬反应[24]。Yang等[25]发现,大麦条纹花叶病毒(Barley stripe mosaic virus,BSMV)的γb蛋白可以通过阻止ATG7与ATG8的互作,从而抑制自噬体的形成。此外,CLCuMuV、TLCYnV、中国番茄黄化曲叶病毒(Tomato yellow leaf curl China virus,TYLCCNV)等双生病毒编码的βC1、C4、C1蛋白也可诱导自噬反应[26-28]。虽然前期研究显示,TYLCV的V3蛋白参与了病毒的移动,并具有沉默抑制子的功能[29-30],但关于双生病毒V3和V4蛋白功能的研究较少,也尚无其参与细胞自噬反应的报道。本研究发现,CCDaV编码的V3和V4蛋白能够诱导细胞自噬的发生,但V3和V4蛋白如何与寄主蛋白互作,从而调控寄主的抗/感病反应的机理还不清楚,今后将进一步开展相关研究。
-
本研究首次证实CCDaV侵染的尤力克柠檬能诱导产生细胞自噬反应,其编码的V3、V4蛋白可能是引起细胞自噬的关键因子。本研究结果为今后深入研究CCDaV与寄主的互作机制提供了重要参考。







 DownLoad:
DownLoad: