-
开放科学(资源服务)标识码(OSID):

-
心理应激是伴侣动物(如犬、猫)在现代驯养环境中面临的主要健康威胁之一[1]。长期暴露于高压环境可引发伴侣动物行为异常、免疫功能抑制及神经退行性病变,严重影响动物福利与生存质量[2-3]。伴侣动物的心理应激源主要包括社交孤立、环境单调性(如长期笼养)、缺乏探索机会或过度限制活动等[4]。其中,慢性束缚应激(Chronic restraint stress,CRS)作为一种典型的可控性心理应激模型,因与家庭宠物被长期限制活动的实际情况高度相似,成为研究伴侣动物应激相关神经损伤的主要范式[5-6]。一直以来,人们对伴侣动物心理应激的关注及研究并不充分,其领域还有大量空白需要填补。因此,预防及治疗动物心理应激具有十分重要的意义。
有研究表明,心理应激可激活下丘脑—垂体—肾上腺轴(Hypothalamic-pituitary-adrenal axis,HPA),促使糖皮质激素异常升高,进而引发系统性病理改变[7]。从行为学层面来看,长期应激导致犬类出现过度理毛(自残倾向)、无目的踱步、社交回避等刻板行为[4];猫科动物则表现为过度警觉、排泄异常或攻击性增强[8]。前人研究发现,CRS能够诱发动物焦虑抑郁样行为,并伴随机体促炎细胞因子和应激激素水平的升高及脑内抗氧化水平的降低[9];另有研究发现,CRS可诱发大鼠大脑海马体萎缩、神经发生减少、血清皮质醇水平升高等现象[10]。因此,开发针对心理应激所致脑损伤的特异性干预药物,对伴侣动物临床医学具有重要价值。
褪黑素(Melatonin,MT)是由脑松果体分泌的天然内源性激素,其独特的脂溶性特征使其可自由穿透血脑屏障,直接调控中枢神经系统功能[11]。其可广泛调控多种生理过程,包括昼夜节律、免疫功能、神经内分泌调节、氧化应激抑制、炎症反应及突触可塑性等[12-13]。研究表明,MT可通过抑制HPA轴的过度激活,降低机体皮质醇浓度,从而起到抗应激作用[14]。同时,作为一种自由基清除剂,MT可直接清除ROS,并通过激活Nrf2通路增强内源性抗氧化酶活性,同时抑制NF-κB信号传导,降低脑组织IL-1β、TNF-α水平[15-16];除此之外,MT可通过MT1/MT2受体介导的PI3K/Akt信号通路,上调海马体BDNF表达,修复突触可塑性损伤[17]。临床上常通过口服方式补充MT改善患者的睡眠障碍;研究发现,腹腔注射MT的生物利用度更高,起效速度更快[18]。
然而,外源添加MT是否能缓解慢性应激所致机体心理应激程度,保护脑区结构和功能的完整性仍未可知。因此,本研究通过对昆明鼠进行为期28 d的CRS建立心理应激模型,并外源添加MT干预,通过行为学测试、ELISA试验、生化鉴定、Nissl染色及免疫荧光染色等方法,评估MT对CRS所致小鼠脑损伤的潜在保护作用,为MT抗伴侣动物心理应激提供理论依据。
HTML
-
CORT、NE、5-HT、MT、DA、IL-1β、IL-6、TNF-α ELISA检测试剂盒购于武汉云克隆科技股份有限公司(货号:SEA133Mu、CEA907Ge、CEA808Ge、CEA908Ge、CEA851Ge、SEA563Mu、SEA079Mu、CEA540Ge);CAT、MDA、SOD活性检测试剂盒,DAPI溶液,甲苯胺蓝染色液,中性树胶购于北京索莱宝科技有限公司(货号:BC0205、BC0025、BC0175、C0065、G3662、G8590);GSH-Px、T-AOC检测试剂盒购于南京建成生物工程研究所(货号:A005-1-2,A015-3-1);樱花OCT冰冻包埋剂购于贵州赛研生物科技有限公司(货号:4583);增强型通用抗体稀释液购于广州亿涛生物科技有限公司(货号:ES-8792);Anti-NeuN Rabbit pAb购于武汉赛维尔生物科技有限公司(货号:GB11138-50);iFluorTM 488 Conjugated Goat anti-rabbit IgG polyclonal Antibody购于杭州华安生物技术有限公司(货号:HA1121);Ultra SensitiveTM SP(鼠/兔)IHC Kit购于福州迈新生物技术开发有限公司(货号:KIT-9710);褪黑素98%购于上海麦克林生化科技股份有限公司(货号:M813985);全自动酶标仪购于广州市番禺区华鑫科技有限公司(型号:ELX808);倒置荧光显微镜购于广州市明美光电技术有限公司(型号:MF52-N);徕卡冷冻切片机购于徕卡显微系统(上海)贸易有限公司(型号:CM1950);艾本德冷冻台式离心机购于成都锦世昌祥科技有限公司(型号:5804R)。
-
本研究严格遵循贵州大学动物试验伦理规范,所有试验操作均经过贵州大学试验动物伦理委员会的批准(批准编号:EAE-GZY-2024-T222)。
试验动物:雄性6周龄昆明鼠48只,体质量(35±2) g,饲养于环境温度(22±2) ℃、环境湿度(50±5)%的试验动物饲养房中。适应7 d后,随机分为对照+生理盐水组(Control with normal saline,CTL+NS)、对照+褪黑激素组(Control with melatonin,CTL+MT)、慢性束缚应激+生理盐水组(Chronic restraint stress with normal saline,CRS+NS)及慢性束缚应激+褪黑素组(Chronic restraint stress with melatonin,CRS+MT)共4组,每组2笼小鼠。每天造模开始前30 min,CTL+NS和CRS+NS组腹腔注射生理盐水(每0.1 mL生理盐水中含有20 μL无水乙醇),CTL+MT及CRS+MT组腹腔注射MT溶液(醇溶,溶解度为50 mg/mL,用生理盐水稀释至10 mg/mL,使用剂量为20 mg/kg)。慢性束缚应激5 h/d(9:00-14:00),束缚期间各组小鼠禁食禁水,试验持续28 d。记录小鼠第0 d、第7 d、第14 d、第21 d和第28 d的体质量变化。造模结束后对小鼠进行眼球采血后脱颈处死。血液样本肝素钠抗凝后于4 ℃保存,脑组织样本处理后于-80 ℃保存。
-
试验第29~31 d,每组随机选取6只小鼠进行强迫游泳测试(Forced swimming test,FST)、悬尾测试(Tail suspension test,TST)、糖水偏好测试(Sucrose preference test,SPT)及旷场测试(Open field test,OFT),参照葛俊铭等的试验方法[19],通过SMART 3.0软件进行分析。
-
血浆及组织中CORT、NE、5-HT、MT、DA、IL-1β、IL-6及TNF-α检测参照武汉云克隆ELISA检测试剂盒说明书进行操作及计算,在酶标仪450 nm波长处测量各孔吸光度值。
-
参照索莱宝、南京建成氧化应激指标活性检测试剂盒说明书,测定小鼠血浆中CAT、MDA、SOD、GSH-Px及T-AOC水平。
-
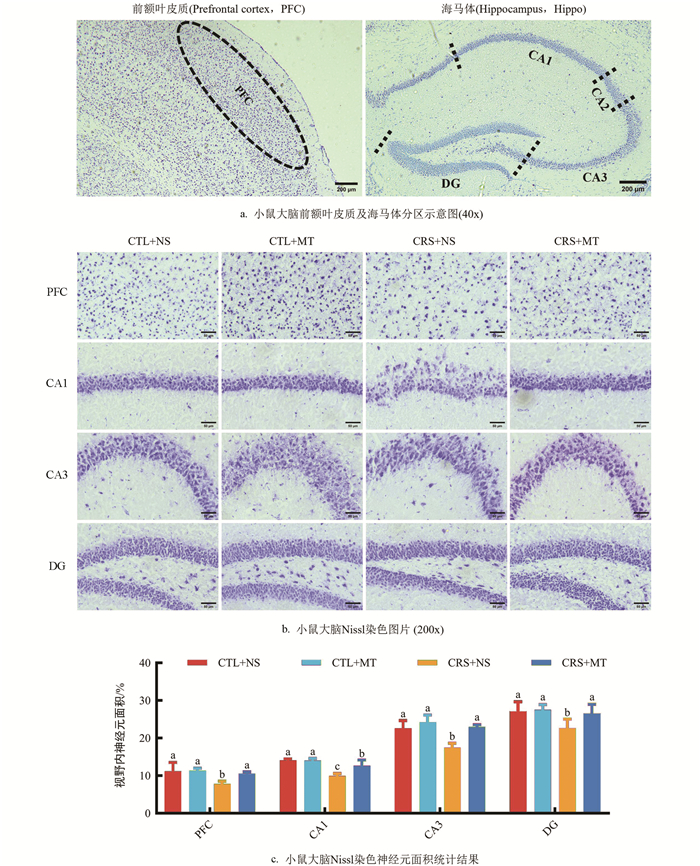
小鼠大脑浸入OCT包埋剂中,于-80 ℃保存。根据小鼠脑区图谱定位前额叶皮质(Prefrontal cortex,PFC)及海马体CA1区、CA3区及DG区位置,作冠状切面。冰冻切片于甲醛中固定10 min,蒸馏水浸洗5 min。甲苯胺蓝染色液于60 ℃烘箱中预热30 min,加盖浸染20 min,蒸馏水浸洗5 min。95%乙醇分化3~5 s。无水乙醇脱水,二甲苯透明,中性树胶封固并拍照记录。使用Fiji Is Just ImageJ v1.50计算视野内神经元面积。
-
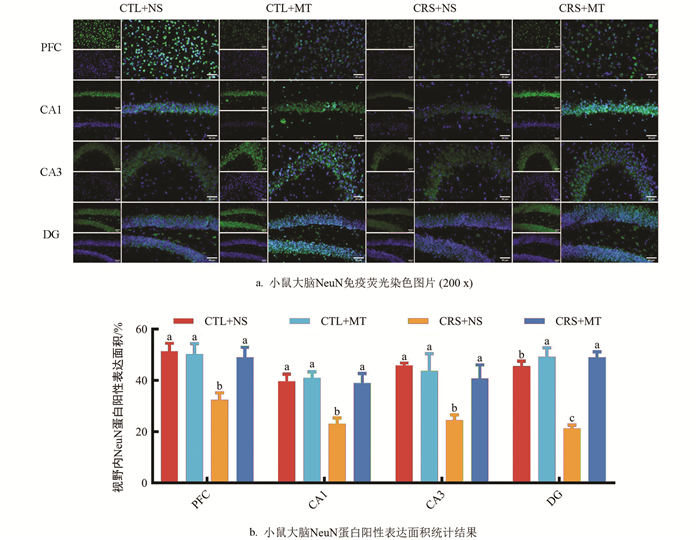
染色前步骤同1.2.5,抗体按照体积比稀释:一抗兔源NeuN∶抗体稀释液(1∶1 000),荧光二抗山羊抗兔IgG∶抗体稀释液(1∶1 000)。参照试剂盒说明书进行操作,滴加DAPI溶液,3 min后在荧光显微镜下拍照记录。使用Fiji Is Just ImageJ v1.50软件计算视野内NeuN蛋白阳性表达面积。
-
使用IBM SPSS 26.0软件对试验数据进行显著性差异分析,通过单因素ANOVA检验组间差异,并进行LSD及邓肯事后检验。组间差异通过字母标记法进行标记,小写字母不同表示组间具有统计学意义(p<0.05),小写字母相同表示组间不具有统计学意义(p>0.05)。使用Graghpad Prism 10.1.2绘图。
1.1. 主要试剂与仪器
1.2. 方法
1.2.1. 试验动物与处理
1.2.2. 行为学测试
1.2.3. ELISA检测
1.2.4. 氧化应激指标检测
1.2.5. 小鼠大脑Nissl染色
1.2.6. 小鼠大脑NeuN免疫荧光染色
1.2.7. 数据统计与分析
-
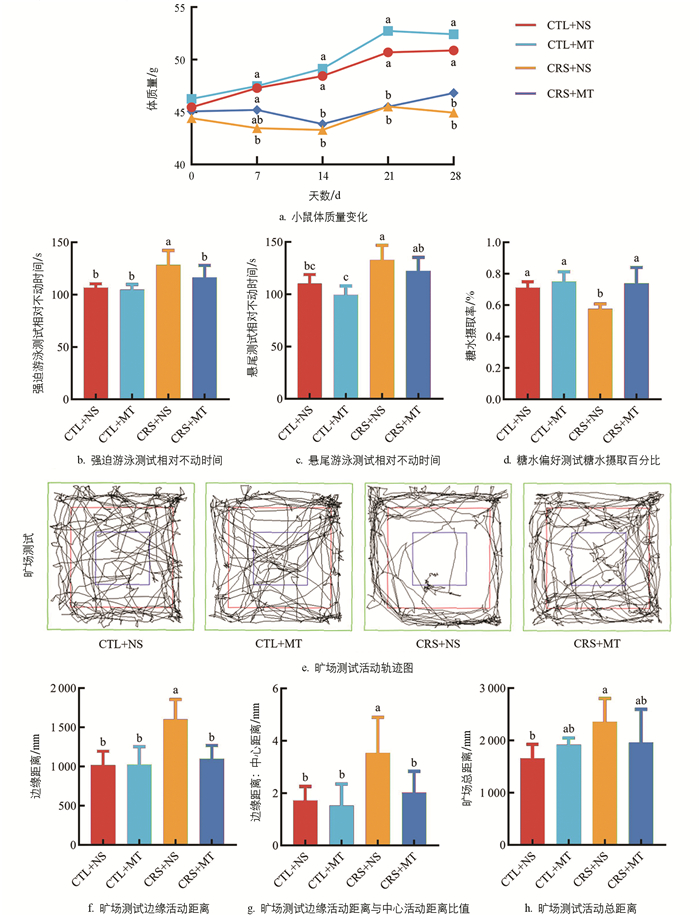
与CTL+NS组相比,CRS+NS组在试验期间第7 d至第28 d的体质量显著下降2.29%~11.68%(p<0.05),添加MT干预后,与CRS+NS组差异不显著(p>0.05,图 1a)。行为学结果显示,相较于CTL+NS组,CRS+NS组的FST及TST相对不动时间分别显著上升20.26%、20.35%(p<0.05),SPT糖水摄取百分比显著下降18.97%(p<0.05);添加MT干预后,CRS+MT组的FST及TST相对不动时间分别显著下降9.44%、7.45%(p<0.05),SPT糖水摄取百分比显著上升28.31%(p<0.05,图 1b-d)。OFT结果显示,CRS+NS组的活动轨迹集中于边缘区域(图 1e)。相较于CTL+NS组和CRS+NS组的边缘活动距离、边缘活动距离与中心活动距离比值及活动总距离显著上升57.13%、106.25%、41.72%(p<0.05);添加MT干预后,CRS+MT组的边缘活动距离、边缘活动距离与中心活动距离比值及活动总距离显著下降31.36%、43.12%、16.82%(p<0.05,图 1f-h)。以上结果提示,CRS导致小鼠出现焦虑抑郁样行为,并伴随体质量增长缓慢现象。添加MT干预缓解了小鼠的焦虑抑郁样行为。
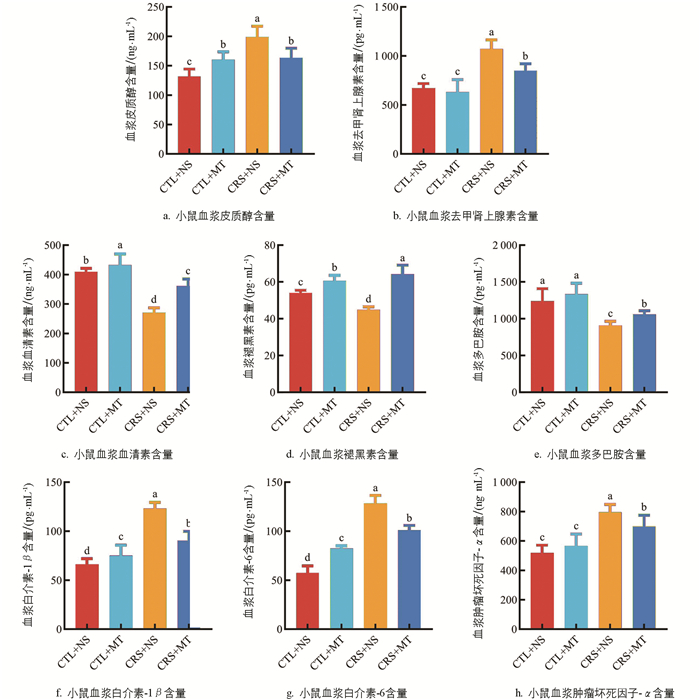
小鼠血浆激素及促炎细胞因子含量变化情况见图 2。ELISA结果表明,与CTL+NS组相比,CRS+NS组血浆的皮质醇(Cortisol,CORT)和去甲肾上腺素(Norepinephrine,NE)比例显著上升51.00%、59.21%(p<0.05);添加MT干预后,CRS+MT组血浆的CORT及NE含量显著下降17.77%、20.94%(p<0.05),提示CRS导致机体处于应激状态,添加MT干预后,机体的应激水平下调。相较于CTL+NS组,CRS+NS组血浆的血清素(5-hydroxytryptamine,5-HT)、MT及多巴胺(Dopamine,DA)含量显著下降33.72%、16.72%、26.75%(p<0.05);添加MT干预后,CRS+MT组血浆的5-HT、MT及DA含量显著上升33.25%、43.10%、16.71%(p<0.05)。
5-HT及DA含量与心理应激严重程度呈负相关,提示CRS导致机体单胺类神经递质水平异常,小鼠伴有心理应激。添加MT干预后,单胺类神经递质水平升高,小鼠的心理应激程度得到缓解。与CTL+NS组相比,CRS+NS组血浆的白介素-1β(Interleukin-1β,IL-1β)、白介素-6(Interleukin-6,IL-6)、肿瘤坏死因子-α(Tumor necrosis factor-α,TNF-α)含量显著上升86.53%、124.36%、53.85%(p<0.05);添加MT干预后,CRS+MT组血浆的IL-1β、IL-6、TNF-α含量显著下降26.64%、21.57%、12.31%(p<0.05)。提示CRS导致机体产生炎性反应,添加MT干预后,机体炎症反应减轻。综上所述,行为学测试结果及ELISA结果共同证明,心理应激小鼠模型及MT干预模型均建立成功。
-
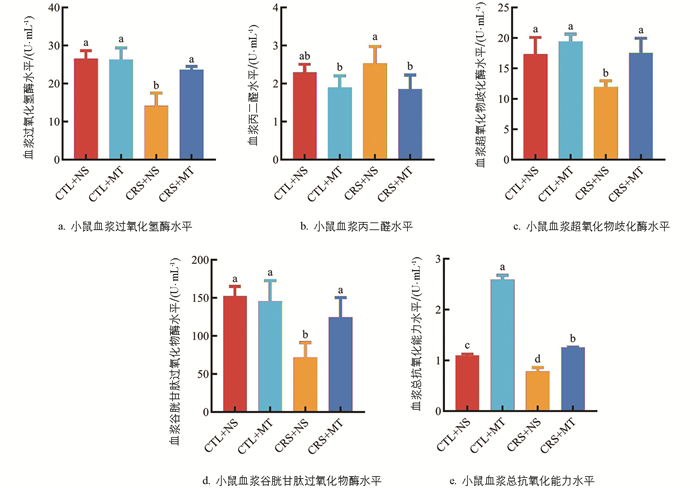
氧化应激指标测定结果表明,相较于CTL+NS组,CRS+NS组血浆的丙二醛(MDA)水平显著上升9.94%(p<0.05),过氧化氢酶(CAT)、超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)及总抗氧化能力水平(T-AOC)显著下降46.64%、30.89%、53.03%、28.57%(p<0.05);添加MT干预后,CRS+MT组血浆的MDA水平显著下降26.81%(p<0.05),CAT、SOD、GSH-Px及T-AOC水平显著上升67.57%、46.77%、73.83%、60.53%(p<0.05,图 3a-e)。提示CRS导致小鼠机体抗氧化能力下降,使其处于氧化应激状态。添加MT干预后,小鼠机体的抗氧化能力上升。
-
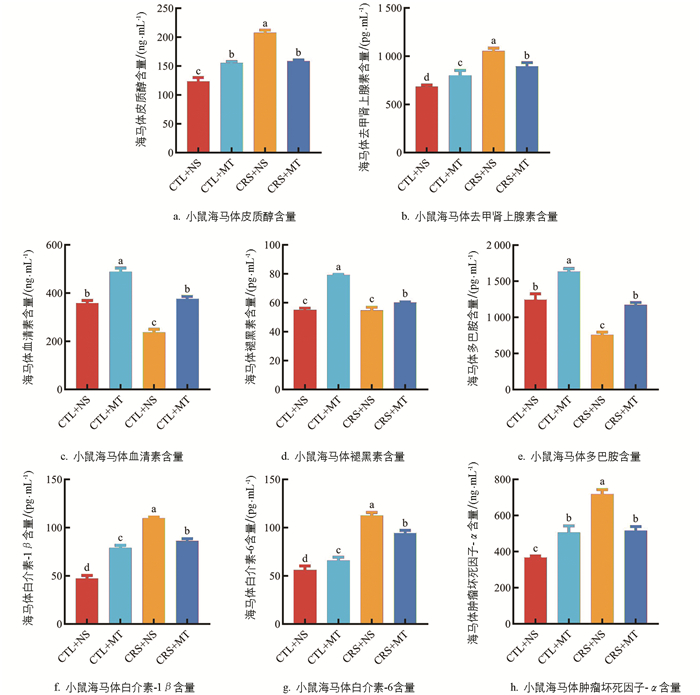
ELISA结果表明,与CTL+NS组相比,CRS+NS组海马体的CORT和NE含量显著上升67.58%、53.55%(p<0.05);添加MT干预后,CRS+MT组海马体的CORT及NE含量显著下降23.72%、15.23%(p<0.05,图 4a、4b)。提示CRS导致海马体的应激激素含量上升,添加MT干预后,海马体的应激激素含量下降。相较于CTL+NS组,CRS+NS组海马体的5-HT及DA含量分别显著下降33.64%、39.25%(p<0.05),MT含量下降不显著(p>0.05);添加MT干预后,CRS+MT组海马体的5-HT、MT及DA含量显著上升58.81%、9.96%、54.82%(p<0.05,图 4c-e)。提示CRS导致海马体的单胺类神经递质含量下降,小鼠伴有心理应激。添加MT干预后,小鼠海马体的单胺类神经递质水平上升,心理应激程度得到缓解。与CTL+NS组相比,CRS+NS组海马体的IL-1β、IL-6、TNF-α含量显著上升133.83%、100.44%、95.39%(p<0.05);添加MT干预后,与CRS+NS组相比,CRS+MT组海马体的IL-1β、IL-6、TNF-α含量显著下降21.42%、16.15%、28.05%(p<0.05,图 4f-h)。提示CRS导致海马体产生神经炎症,促炎细胞因子含量上升,添加MT干预后,海马体的神经炎症减轻,促炎细胞因子含量下降。
-
Nissl染色结果表明(图 5a),相较于CTL+NS组,CRS+NS组PFC区、CA1区、CA3区、DG区的视野内神经元面积占比显著下降29.80%、29.53%、22.65%、16.52%(p<0.05);添加MT干预后,CRS+MT组PFC区、CA1区、CA3区、DG区的视野内神经元面积占比显著上升34.56%、27.28%、31.69%、17.32%(p<0.05,图 5b,5c)。提示CRS导致小鼠大脑PFC及海马体神经元受到损伤,神经元数量、密度、大小显著下降,细胞排布紊乱,伴有形态异常。相反,添加MT干预后,各区域神经元损伤程度减轻。
-
NeuN染色结果表明,相较于CTL+NS组,CRS+NS组PFC区、CA1区、CA3区、DG区的视野内NeuN蛋白阳性表达面积占比显著下降37.00%、41.83%、46.50%、53.32%(p<0.05);添加MT干预后,CRS+MT组PFC区、CA1区、CA3区、DG区的NeuN蛋白阳性表达面积占比显著上升51.22%、69.05%、66.37%、130.57%(p<0.05,图 6a、6b)。提示CRS导致小鼠大脑PFC及海马体区域NeuN蛋白阳性表达面积降低,神经发生减少。相反,添加MT干预后,各区域内NeuN蛋白阳性表达面积升高。
2.1. 心理应激小鼠模型及MT干预小鼠模型建立验证分析
2.2. MT干预对CRS小鼠血浆氧化应激指标的影响
2.3. MT干预对CRS小鼠海马体激素及促炎细胞因子含量的影响
2.4. MT干预对CRS小鼠大脑神经元面积的影响
2.5. MT干预对CRS小鼠大脑NeuN蛋白阳性表达面积的影响
-
本研究对小鼠进行了为期28 d的CRS,并外源添加MT干预。结果表明,与CTL+NS组相比,CRS+NS组在试验期间第7 d至第28 d的体质量显著下降,添加MT干预后,CRS+MT组的体质量变化并不显著。在造模第29~31 d,对小鼠进行了4项行为学测试。结果表明,CRS+NS组小鼠的FST及TST相对不动时间、OFT边缘活动距离、边缘活动距离与中心活动距离比值及活动总距离显著上升,SPT糖水摄取百分比显著下降,提示CRS导致了小鼠焦虑抑郁样行为的发生。Deng等[20]研究发现,CRS导致小鼠FST相对不动时间显著上升,SPT糖水偏好百分比显著下降等,这与本研究结果一致,提示造模成功。然而,前人研究发现,CRS大鼠的OFT活动总距离显著下降,这与我们的试验结果不符,可能与CRS的持续时间、试验动物的品种有关[21]。
CRS通过激活HPA轴,导致CORT的大量释放,其神经毒性使PFC和海马体的神经元整体萎缩,突触抑制,神经发生等减少。此外,CRS还会导致机体的体质量增长减缓,免疫功能障碍,氧化应激水平升高[7]。因此,本研究进一步测定了小鼠血浆及海马体中应激激素、神经递质的含量及抗氧化水平。结果表明,CRS+NS组小鼠的血浆和海马体中,CORT、NE含量显著上升,5-HT、DA和血浆中MT的含量显著降低,而IL-1β、IL-6、TNF-α的含量显著上升,提示CRS导致小鼠机体处于应激状态,中枢神经递质紊乱,免疫功能障碍。CRS+NS组小鼠血浆的MDA水平显著上升,CAT、SOD、GSH-Px及T-AOC水平显著下降,提示CRS导致小鼠机体抗氧化能力下降,使其处于氧化应激状态。Ye等[22]发现,由CRS诱导的小鼠血浆和海马体中5-HT含量显著下降,IL-1β、TNF-α含量显著上升,这与我们的研究结果一致。CRS损伤了患畜大脑的情绪调节功能,诱导了心理应激的发生。因此,进一步通过组织学方法检测了小鼠大脑结构和功能的完整性。染色结果表明,CRS+NS组小鼠大脑PFC及海马体神经元面积占比显著下降,NeuN蛋白阳性表达面积占比显著下降;神经元大部分呈现体积缩小,细胞数量减少和细胞排布紊乱。大量研究表明,长期应激会导致PFC和海马体的神经元整体萎缩以及突触抑制,免疫炎症反应增强,小胶质细胞被过度激活,产生大量促炎细胞因子,阻碍神经发生[23]。
本研究发现CRS不仅导致单胺类神经递质分泌异常,还导致了血浆内MT水平的下降。由于MT与5-HT及DA的特殊关系,认为MT含量过低或许与焦虑抑郁样行为的发生有关。因此,本研究通过腹腔注射MT,对CRS小鼠血浆中MT含量进行回补,对其焦虑抑郁样行为、机体应激激素水平、单胺类神经递质水平以及促炎细胞因子水平等进行了测定。结果表明,MT能够显著缓解CRS小鼠的焦虑抑郁样行为。添加MT干预后,CRS+MT组小鼠的FST及TST相对不动时间、OFT边缘活动距离、边缘活动距离与中心活动距离比值及活动总距离显著下降,SPT糖水摄取百分比显著上升,焦虑抑郁样行为得到缓解,这与前人的研究结果一致[24]。进一步研究表明,添加MT逆转了由CRS诱导的血浆和海马体中CORT、NE、IL-1β、IL-6、TNF-α含量升高,并使得5-HT、MT和DA含量恢复到正常水平,与CTL+NS组无显著差异。前人研究结果表明,在LPS诱导小鼠抑郁样行为试验中,MT治疗显著抑制促炎细胞因子IL-1β、IL-6、TNF-α的水平,抑制NF-κB磷酸化及神经胶质细胞的活化,从而预防神经炎症[25],这与我们的研究结果类似。氧化应激指标结果表明,添加MT干预显著降低了CRS小鼠机体的氧化应激水平。组织学试验结果表明,添加MT干预逆转了由CRS诱导的小鼠大脑神经元损伤及NeuN蛋白表达降低,提示MT对CRS造成的神经元损伤具有一定的保护作用。有研究结果证实,MT能够通过降低海马体中的脂质过氧化和NO水平,增强SOD和CAT活性,减弱铜引起大鼠海马体氧化应激,从而改善铜诱导的焦虑及抑郁样行为[26]。本研究中,MT或通过抑制氧化应激和炎症反应,保护脑部结构和功能完整性,抑制CRS致小鼠心理应激及焦虑抑郁样行为的发生。
MT具有抗应激、抗氧化及抗炎作用,且对于单胺类神经递质具有调节作用。应激状态下,MT可对HPA轴产生显著的抑制性作用,降低CORT及NE的释放[14]。作为一种自由基清除剂,MT抗氧化作用介导了神经保护作用。MT能够刺激神经可塑性的所有阶段,其发挥作用主要通过M1、M2受体及非受体途径进行。本研究结果证实,MT通过下调应激激素,提升机体抗氧化能力;下调促炎细胞因子水平,发挥了神经保护作用。然而,其作用机制仍需进一步探讨。前人研究发现,MT通过激活Nrf2通路、BDNF/Tr-kB通路等发挥抗氧化作用,激活抗氧化物酶,降低机体氧化应激水平[27-28];MT通过调节COX-2表达,抑制NF-κB信号通路等发挥抗炎作用,下调IL-1β、IL-6、TNF-α等促炎细胞因子水平[29-30];此外,MT还通过激活脑内M1、M2受体,调节中枢神经递质[12]。MT在抗CRS所致心理应激中的作用机制非常复杂,因此,对于本研究中MT发挥作用的分子机制及胞内信号途径,还需进一步探索。
综上所述,本研究发现CRS导致小鼠表现出焦虑抑郁样行为,机体发生应激反应,伴有氧化应激水平升高、单胺类神经递质紊乱、促炎细胞因子水平升高及大脑神经元损伤等。MT通过其抗应激、抗氧化、抗炎及调节单胺类神经递质作用,缓解了小鼠的心理应激,发挥神经保护作用。研究表明,MT对CRS致小鼠脑损伤具有潜在的治疗作用,且无明显副作用,为MT抗伴侣动物心理应激提供了理论依据。
-
研究表明,CRS诱导小鼠出现焦虑抑郁样行为,致小鼠发生心理应激,机体处于氧化应激状态,免疫功能下降,神经递质紊乱,神经功能异常。添加MT干预后,小鼠机体的应激水平下降,炎症反应减轻,神经功能异常得到缓解,脑损伤减轻。






 DownLoad:
DownLoad: