-
近年来肿瘤的产生和治疗在生物和医学等众多领域引起了十分广泛的关注. 不同的数学动力学模型被构建出来研究肿瘤与体内免疫系统之间的相互作用[1-4]. 为了更好地降低肿瘤导致的死亡率或延长患者的寿命,研究不同治疗方案的效果是非常有意义的. 常见的肿瘤治疗方案有化疗、免疫治疗以及放射治疗[5-6]. 考虑到肿瘤激活免疫效应细胞需要一定时间,文献[7-8]研究了免疫激发离散时滞对肿瘤免疫系统动力学性态的影响,注意到肿瘤激发免疫反应所需要的时间往往是在某一个值附近波动,因而考虑分布时滞在一定条件下会更加准确.
我们指出相关研究大多假设免疫细胞完全由肿瘤细胞激发,得到的相应模型往往表明体内免疫细胞在一定条件下可能会无限增长. 实际上体内免疫系统由多类细胞构成,有的免疫细胞,如巨噬细胞,在没有肿瘤细胞的条件下也会自然分泌和增长. 因而在考虑免疫细胞由肿瘤激发生成的同时假设其依据logistic自然增长也是非常有意义的,这在相关研究中还不多见. 同时,文献[10]提出循环淋巴细胞可以用来衡量免疫系统的健康状况并且促进免疫效应细胞的增长. 基于上述讨论,本文在经典的肿瘤免疫系统中加入循环淋巴细胞,同时考虑了化疗、免疫治疗及分布时滞,并假设免疫细胞依据logistic自然增长,最终建立如下动力学模型:
其中:
$G=\int_{0}^{\infty} K(s) T(t-s) \mathrm{d} s ; T(t), I(t), C(t)$ 分别表示t时刻肿瘤细胞、免疫细胞和循环淋巴细胞的数量;M1(t)和M2(t)分别代表t时刻血液中的化疗药物和免疫治疗药物浓度;u1(t)和u2(t)分别代表由外部输入的化疗药物和免疫治疗药物的数量;a1和a2分别为肿瘤细胞和免疫细胞的内稟增长率;$\frac{1}{b_{i}}(i=1, 2)$ 为环境容纳量;m为免疫细胞对肿瘤细胞的杀死率;n为肿瘤细胞对免疫细胞的激活率;k为免疫治疗对免疫细胞的激活率;α1和α2分别为循环淋巴细胞的转化率和自然转化率;β为循环淋巴细胞的死亡率;qi(i=1, 2, 3)为化疗药物对相应细胞的杀伤率;γi(i=1, 2)为化疗药物和免疫治疗药物的衰减率. 本文假设肿瘤激活免疫细胞进行免疫反应所需的时间依概率密度函数K(s)分布,满足K(s)≥0且$\int_{0}^{\infty} K(s) \mathrm{d} s=1$ . 为了便于研究系统(1)的动力学行为,假设输入的化疗药物u1(t)和免疫治疗药物u2(t)均为常数,即u1(t)=u1, u2(t)=u2.设Φ∈$\mathscr{C}$((-∞, 0], $\mathbb{R}^{+}$), 由生物学意义给定系统(1)的非负初始条件如下:
HTML
-
定理1 系统(1)的所有解T(t), I(t), C(t), M1(t), M2(t)是非负且有界的.
证 对系统(1)显然有:
由非负初始条件(2)知:T(t), C(t), M1(t), M2(t)均是非负的. 接着根据C(t)的非负性,可以得出
从而知
故系统(1)的所有解是非负的.
下面证明系统的有界性. 首先,由系统(1)的第一个和第三个方程知:
运用比较原理可知:
类似地,由系统(1)的第四个和第五个方程知:
至此证明了T(t), C(t), M1(t), M2(t)是有界的. 接着,证明I(t)的有界性. 由系统(1)的第二个方程以及T(t), C(t), M1(t), M2(t)的有界性知存在正常数K1和K2使得:
从而知I(t)也是有界的. 定理证毕.
-
本节讨论免疫激发分布时滞为零时系统(1)的动力学性态. 此时系统(1)变为如下常微分方程:
-
定理2 (i)系统(3)总存在无肿瘤平衡点E0=(0, I0, C0, M10, M20).
(ii) 如果
$I_{0}>\frac{1}{m}\left(a_{1}-\frac{q_{1} u_{1}}{\gamma_{1}}\right)$ , 则系统(3)的无肿瘤平衡点E0是局部渐近稳定的.证 (i)首先由系统(3)的后3个方程可得:
将C0, M10, M20分别代入到系统(3)的第二个方程,可得:
其中,
由A1 > 0, A3 < 0知方程(4)存在正根
故系统(3)总存在无肿瘤平衡点E0=(0, I0, C0, M10, M20).
(ii) 为了研究无肿瘤平衡点E0的局部稳定性,计算系统(3)在平衡点E0处的雅可比矩阵为:
从而对应特征方程的根分别为:
显然,特征值λ1, λ2, λ3, λ5是负的;当λ4 < 0时,即
$I_{0}>\frac{1}{m}\left(a_{1}-\frac{q_{1} u_{1}}{\gamma_{1}}\right)$ 成立,则无肿瘤平衡点E0是局部渐近稳定的. 定理证毕. -
定理3 (i) 如果
$I^{*}<\frac{1}{m}\left(a_{1}-\frac{q_{1} u_{1}}{\gamma_{1}}\right)$ , 则系统(3)存在正平衡点E*=(T*, I*, C*, M1*, M2*).(ii) 系统(3)的正平衡点E*是局部渐近稳定的.
证 (i) 如果系统(3)存在正平衡点E*=(T*, I*, C*, M1*, M2*), 则有:
且I*应满足下列方程:
其中:
由B1 > 0, B3 < 0知方程(5)存在正根
故当T* > 0时,即
$I^{*}<\frac{1}{m}\left(a_{1}-\frac{q_{1} u_{1}}{\gamma_{1}}\right)$ , 系统(3)存在正平衡点E*=(T*, I*, C*, M1*, M2*).(ii) 下面研究正平衡点E*的局部稳定性,计算系统(3)在平衡点E*处的雅可比矩阵为:
从而系统(3)在E*处的特征方程为:
其中
显然特征方程(6)有3个负的特征根:
另外两个特征根λ4和λ5由下述方程决定:
可得
从而知λ4和λ5均为具有负实部的特征根. 故正平衡点E*是局部渐近稳定的. 定理证毕.
由定理2和定理3, 我们定义:
则可以进一步得到如下结论.
定理4 (i) 假设
$u_{1}>\frac{a_{1} \gamma_{1}}{q_{1}}$ , 则系统(3)存在唯一无肿瘤平衡点E0且局部渐近稳定.(ii) 假设
$u_{1}<\frac{a_{1} \gamma_{1}}{q_{1}}$ . 如果u2 > Q(u1), 则系统(3)的无肿瘤平衡点E0局部渐近稳定;如果u2 < Q(u1), 无肿瘤平衡点E0不稳定,而正平衡点E*存在且局部渐近稳定.证 (i) 注意到
$a_{1}-\frac{q_{1} u_{1}}{\gamma_{1}}<0$ 等价于$u_{1}>\frac{a_{1} \gamma_{1}}{q_{1}}$ , 由定理2和定理3可知(i)成立.(ii) 如果
$a_{1}-\frac{q_{1} u_{1}}{\gamma_{1}}>0$ , 通过计算可以得到:故由定理2和定理3可知(ii)成立.
注1 由定理4可知,只要化疗药物浓度足够大,即
$u_{1}>\frac{a_{1} \gamma_{1}}{q_{1}}$ , 肿瘤可以直接被清除;注意到化疗的副作用,可以知道u1的增加会降低免疫细胞的浓度. 如果结合化疗和免疫治疗,使得u2 > Q(u1), 则可能在$u_{1}<\frac{a_{1} \gamma_{1}}{q_{1}}$ 条件下,即保持较高免疫水平的条件下控制并清除肿瘤.
2.1. 无肿瘤平衡点分析
2.2. 正平衡点分析
-
本节讨论系统(1)在免疫激发分布时滞不为零的情况下正平衡点处的稳定性. 参考文献[9], 本文取核函数为弱核,即K(s)=θe-θs, θ > 0, 则相应的平均时滞为:
下面研究Hopf分支的情况. 首先引入新的变量:
系统(1)变为如下等价的常微分方程:
注意到时滞不改变平衡点的存在性,所以系统(3)的正平衡点等价于系统(7)的正平衡点
$\widetilde{E}=(\widetilde{T}, \tilde{I}, \tilde{Y}, \widetilde{C}, \widetilde{M}_{1}, \widetilde{M}_{2})$ , 其中$\widetilde{T}=\widetilde{Y}$ . 此时将系统(7)在正平衡点$\widetilde{E}$ 处线性化可得:从而可得系统(8)在正平衡点
$\widetilde{E}$ 处特征方程对应的根均为负根;其余3个特征根由下列方程决定:
其中:
注意到Li(θ) > 0(i=1, 2, 3), 由Hurwitz判据可知,方程(9)的3个根都具有负实部,即正平衡点
$\widetilde{E}$ 局部渐近稳定的充要条件是代入Li(θ) > 0(i=1, 2, 3)计算可得
其中:
显然H1 > 0, H3 > 0.
令Δ=H22-4H1H3, 由此可知:当Δ≤0时,Q(θ)≥0在θ > 0上恒成立,则正平衡点
$\widetilde{E}$ 对所有时滞都局部渐近稳定. 当Δ > 0时,定义:若H2 > 0, Q(θ) > 0在θ > 0上恒成立,此时正平衡点
$\widetilde{E}$ 对所有时滞都局部渐近稳定;若H2 < 0, 当0 < θ < θcr1或θ > θcr2时$\widetilde{E}$ 局部渐近稳定,当θcr1 < θ < θcr2时正平衡点$\widetilde{E}$ 不稳定.当θ=θcri(i=1, 2)时,有Q(θcri)=0, 易知方程(9)存在一对纯虚根. 接下来根据文献[12], 只需验证Hopf分支发生的横截条件. 由(10)式可以得到:
故系统(1)在θ=θcri(i=1, 2)处发生Hopf分支. 依据上述讨论,给出如下定理.
定理5 (i) 如果Δ≤0, 则正平衡点
$\widetilde{E}$ 对所有时滞都局部渐近稳定.(ii) 如果Δ > 0且H2 > 0, 则正平衡点
$\widetilde{E}$ 对所有时滞都局部渐近稳定.(iii) 如果Δ > 0且H2 < 0, 则当0 < θ < θcr1或θ > θcr2时,正平衡点
$\widetilde{E}$ 局部渐近稳定;当θcr1 < θ < θcr2时,正平衡点$\widetilde{E}$ 不稳定且在θ=θcri(i=1, 2)处发生Hopf分支.注2 由定理5可以看到当平均时滞处于中间水平时系统会产生周期解,该结果与文献[9]中的结论完全不同. 在文献[9]中只存在一个Hopf分支点,并且当平均时滞较小时存在周期解. 我们认为该差异产生的原因主要在于假设免疫细胞由肿瘤激发生成的同时还依据logistic自然增长,说明免疫细胞的自然增长对该系统动力学性态有重要影响.
-
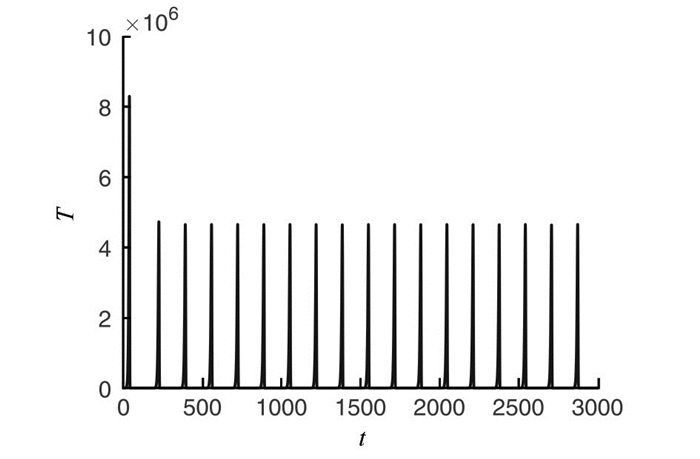
为了验证上述理论研究结果,参考文献[11]取定一组参数如下:a1=0.43, b1=1.02×10-9, m=6.41×10-12, a2=5×10-2, b2=1.2×10-11, n=2×10-7, k=6.25×10-6, q1=0.8, q2=q3=0.6, α1=2.08×10-2, α2=7.50×108, β=1.2×10-3, γ1=0.9, γ2=1, u1=0.115, u2=0.113, 计算可得系统(7)存在如下正平衡点:
$\widetilde{E}=\left(2.68 \times 10^{5}, 5.12 \times 10^{10}, 2.68 \times 10^{5}, 9.63 \times 10^{9}, 0.13, 0.11\right)$ . 通过计算可得临界值θcr1=8.66×10-6, θcr2=0.47. 分别取θ1=8.3×10-6, θ2=0.6, 由定理5可知,正平衡点$\widetilde{E}$ 此时皆局部渐近稳定(图 1); 取θ=0.2时θcr1 < θ < θcr2, 则由定理5可知,正平衡点$\widetilde{E}$ 失去稳定性,系统产生Hopf分支出现周期解(图 2).







 DownLoad:
DownLoad: