-
癌症已发展为影响人类健康的3大疾病之一,仅次于心血管病. 根据国家疾病中心数据显示,在2016年内,中国乳腺癌发病为273 000例,高居女性恶性肿瘤之首[1],因而研究如何防治乳腺癌已成为临床医学的主要任务之一.
流行病学调查显示,定期进行中等强度的体育锻炼与降低多种癌症的风险相关,其中包括乳腺癌、结肠癌和子宫内膜癌等[2-4]. 有规律的运动可以显著降低癌症高风险人群中的癌症危险生物标志物[5-6],同时,有氧运动可以改善患癌后生活质量,并可使特定癌症死亡率和全因死亡率降低30%~50%[7-9]. 尽管有氧运动能够降低患癌风险和改善预后,但少有研究关注有氧运动干预对肿瘤生长的影响[10]. 有氧运动干预抑制肿瘤生长的效果受动物品系、运动方式、运动时间和强度等多种因素的影响[11-16].
细胞凋亡是细胞基本生理活动之一,与肿瘤的发生和发展密切相关. 肿瘤“微环境”中存在的炎症因子可使NF-κB信号通路过度激活,抑制凋亡蛋白的表达,影响癌症正常细胞凋亡,进而加速肿瘤生长[17]. 因此,寻找抑制NF-κB信号通路过度激活的方法和药物成为肿瘤学研究的重要内容.
本实验以原位移植4T1乳腺癌细胞的Balb/C小鼠为模型,研究了中等强度有氧运动对肿瘤生长的影响,并通过观察肿瘤组织内NF-κB通路关键蛋白变化进行机制探索.
HTML
-
SPF级Balb/C小鼠,雌性,6~8周龄,体质量(18±2) g,购于重庆瀚东实验动物公司,动物合格证号SCXK(京)2017-0003. 小鼠饲养于西南大学药学院动物实验中心,每笼5只群养,自由饮食、饮水,室温23~24 ℃,湿度(50±10)%,人工12 h/12 h昼夜节律光照. 每2 d更换一次鼠料及清理饲养笼具. 4T1小鼠乳腺癌细胞购于中国科学院上海细胞库,根据常规贴壁细胞的方式培养.
-
DB030型六道动物实验跑台,北京智鼠多宝生物科技有限公司;TGL-16C台式离心机,美国Sigma公司;AJC-0501-P纯水仪,WH-2微型漩涡混合器,美国安胜;TL-420D水浴,姜堰市天利医疗设备有限公司;Rt2100c酶标检测仪,Rayto.
-
1640培养基,BCA蛋白定量检测试剂盒(G2026)购于Servicebio;蛋白Marker(26616)购于Therm(Fermentas);GAPDH (GB12002)购于Servicebio;MouseIL-1β ELISA kit(EK201B1/2),Enzyme-linked Immunosorbent Assay Kit For Estradiol(CEA461Ge)购于武汉优尔生科技股份有限公司.
-
将4T1小鼠乳腺癌细胞放置在含有10%胎牛血清的1640培养基中,于5%-CO2,37 ℃条件培养箱中. 取对数期细胞用胰蛋白酶消化,PBS清洗并改变细胞浓度为1×106个/mL. 于小鼠右腋下皮下注射4T1乳腺癌细胞悬液0.2 mL,自然生长7 d后测量肿瘤大于2 mm×2 mm时,判断造模成功[18].
-
接种前,将小鼠随机分成正常对照(NC)组10只,运动对照(NCE)组10只,模型组20只. 造模成功后,分模型运动组(TCE)与模型对照组(TC)各10只. 各组正常进食饮水,对20只NCE和TCE组小鼠进行中等强度有氧运动干预[19]. 干预方式:设置跑台速度10 m/min,0坡度,60 min/次,5次/周(周1至周5,晚间6:00-7:30),持续28 d. 为消除噪声干扰,将NC和TC组小鼠放在跑步机旁边但不运动.
-
造模成功后,每3 d记录一次小鼠体质量和瘤体积(V),后者测量方式:分别用游标卡尺测量肿瘤长度和宽度,然后使用公式V=0.5×W2×L(W是瘤宽,L是瘤长)计算. 30 d后,将小鼠处死并收集肿瘤,称质量,做好记录并于-80 ℃条件存放.
-
30 d后,在处死小鼠前,从眼睑取血,离心收集血清,-80 ℃条件下存放. 严格按照ELISA试剂盒步骤分别测量IL-1β和E2质量浓度.
-
用组织匀浆器和组织蛋白裂解液对小鼠肿瘤组织进行破碎,离心并收集蛋白样品. BAC法测定蛋白浓度后,进行SDS-PAGE电泳. 转膜、封闭,洗膜后加入1∶1 000稀释的一抗(NF-κBP65,IκBα,GAPDH),4 ℃孵育过夜. TBST洗膜后加入1∶2 000稀释的HRP标记的二抗,室温孵育2 h后洗膜,ECL化学发光液显色,凝胶成像系统拍照,Image J软件进行灰度处理.
-
所得数据由Spss 19.0软件中的单因素方差分析进行处理,用x±s进行表示,并用Origin 8.0软件作图.
1.1. 动物与细胞株
1.2. 主要仪器
1.3. 主要试剂
1.4. 造模
1.5. 分组及运动干预方案
1.6. 动物体质量与瘤质量检测
1.7. 血清内IL-1β,E2指标检测
1.8. Western blot检测小鼠瘤组织中蛋白表达
1.9. 统计学处理
-
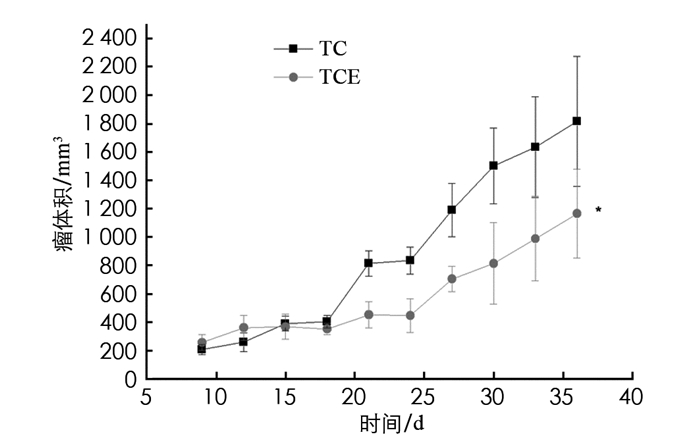
运动干预结束后48 h,迅速完成取材,并将肿瘤组织拍照(部分有破损,每组取8个完整肿瘤组织来拍照). 较TC组,经有氧运动干预后TCE组小鼠肿瘤体积增长曲线更为平缓(图 1),且体积明显变小(图 2). 结合表 1,TCE组最后1次测量的瘤体积及瘤质量均小于TC组,差异有统计学意义(p<0.05). 由此可见,中等强度有氧运动对4T1乳腺癌肿瘤生长有显著的抑制作用.
-
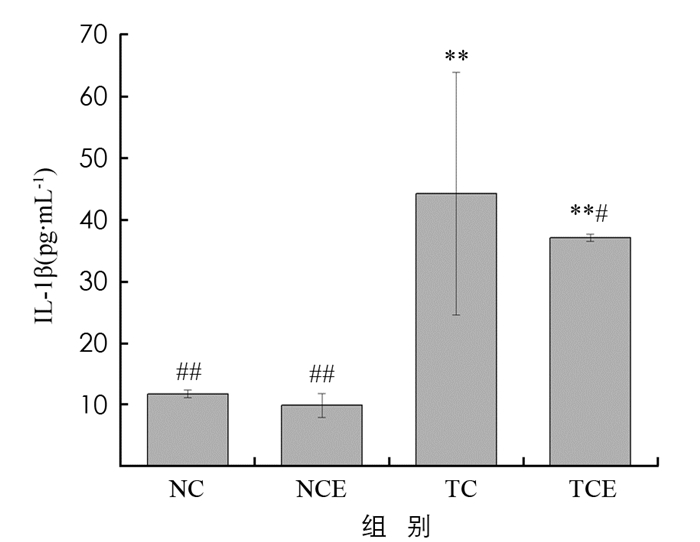
血清中E2和IL-1β质量浓度同乳腺癌的出现两者密切相关,肿瘤能够刺激机体产生炎症反应,而IL-1β是重要的炎症调节因子. 图 3显示,TC组和TCE组的IL-1β水平明显高于NC组(p<0.01). 而与TC组相比,中等强度有氧运动干预的TCE组小鼠体内的IL-1β质量浓度明显下降了16%,并达到统计学意义(p<0.05). 说明中等强度有氧运动干预能够明显改善4T1乳腺癌小鼠体内IL-1β质量浓度,减轻炎症反应.
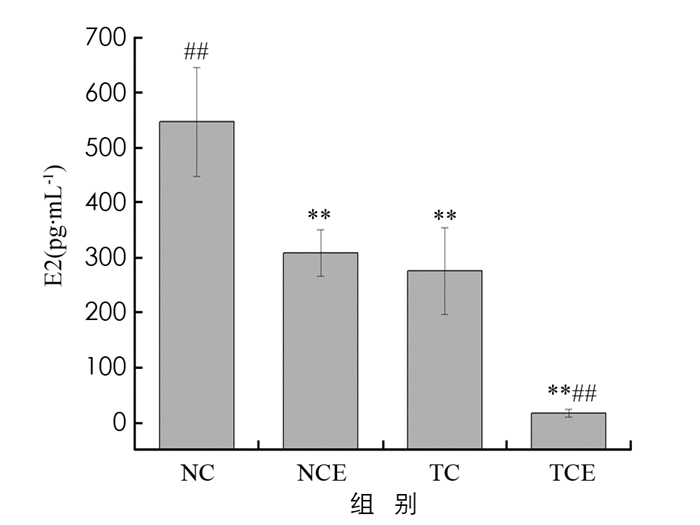
图 4显示,与NC组相比,NCE,TC,TCE组小鼠体内E2质量浓度分别下降了44%,50%,97%,差异有统计学意义(p<0.01). 经中等强度有氧运动干预后TCE组内E2质量浓度比TC组下降了94%,差异有统计学意义(p<0.01). E2是体内重要的雌激素,较高的表达水平会促进乳腺癌的发展,此结果表明中等强度有氧运动干预可以显著降低E2的质量浓度,从而抑制乳腺癌的发生.
-
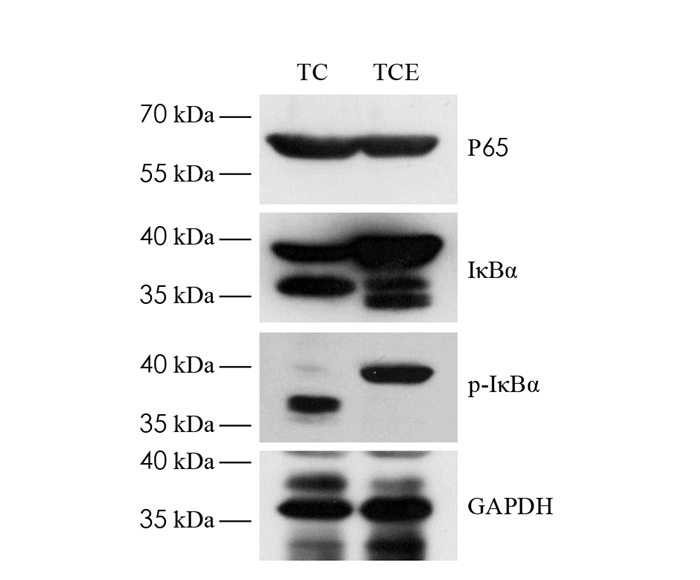
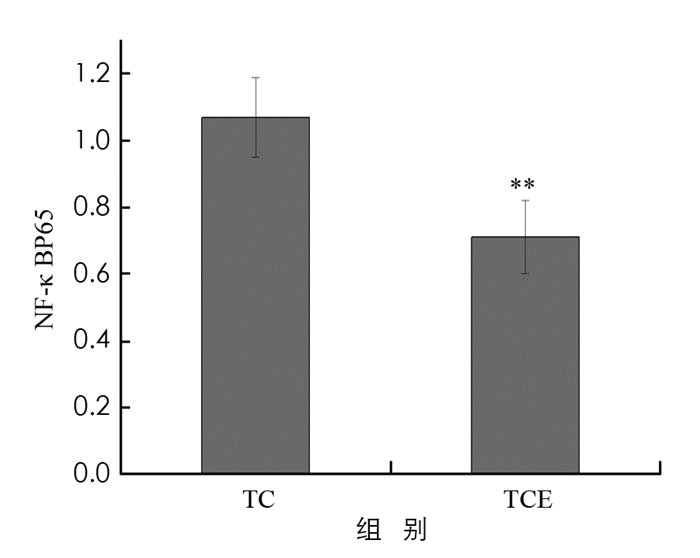
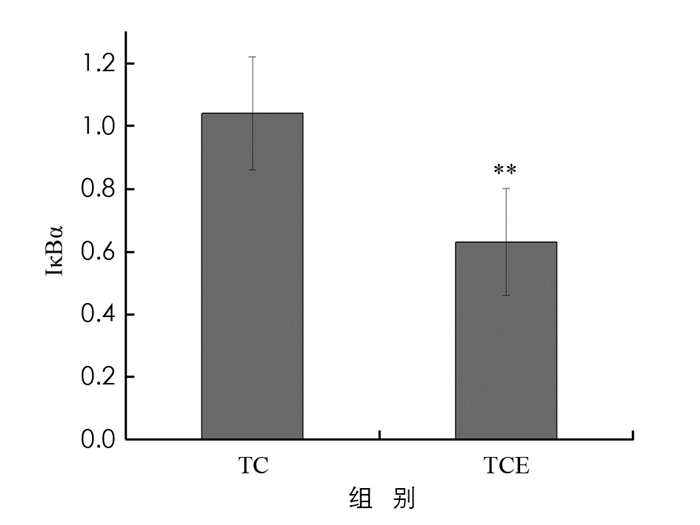
在乳腺肿瘤组织中普遍存在NF-κB激活现象,NF-κB在乳腺癌的发生、发展及转移中具有重要作用[20],其中,NF-κBP65,IκBα蛋白可以减少NF-κB入核和抑制转录过程的发生,从而抑制NF-κB通路的过度激活. 本实验利用Western blot检测小鼠瘤组织中NF-κBP65,IκBα蛋白表达变化(图 5).
图 6显示,TCE组小鼠肿瘤组织内NF-κBP65蛋白表达比TC组下调了34%,差异有统计学意义(p < 0.01). 结合图 7,较TC组,TCE组小鼠肿瘤组织中IκBα蛋白表达显著下调了39%,差异有统计学意义(p < 0.01). 结果表明,中等强度有氧运动干预能够显著降低NF-κBP65和IκBα蛋白表达,抑制NF-κB信号通路过度激活.
2.1. 有氧运动干预对小鼠肿瘤生长的影响
2.2. 有氧运动干预对小鼠血清IL-1β,E2指标的影响
2.3. 中等强度有氧运动干预对小鼠肿瘤组织NF-κB信号通路相关蛋白的影响
-
乳腺癌对于女性来说是最致命的恶性肿瘤之一,全球乳腺癌发病率呈现快速增长的趋势,因而探寻新的治疗手段对人类具有重要的意义.
目前,已开发出自发性、诱发性、移植性、远处转移和转基因等乳腺癌小鼠模型用作实验研究. 4T1乳腺癌细胞形成肿瘤具有血管丰富、免疫原性低、早期就可发生远处易感器官转移等特点,其生长特性与人类乳腺癌相似度极高. 有证据表明,4T1小鼠乳腺癌细胞系移植入免疫功能正常的同品系小鼠体内,从而模拟人乳腺癌发展和转移情况,此种方法比将人乳腺癌细胞系(异种移植物)移植入免疫功能缺陷小鼠体内来观察肿瘤发展和转移情况更加有效,并得到了广泛认可[21]. 因此,本研究采用4T1细胞原位移植模型,于小鼠右腋下皮下注射4T1乳腺癌细胞悬液0.2 mL,所有小鼠在7 d后均检测到肿瘤体积大于2 mm×2 mm,显示此次实验造模成功.
有氧运动干预可以显著降低多种乳腺癌的患病风险、改善生活质量,但有氧运动干预对乳腺癌成瘤后的影响研究相对较少,且效果并不明显,其可能与单次有氧运动干预时间相关. 王斌等的研究发现经30 min/次单纯有氧运动干预后原位接种4T1细胞的Balb/C小鼠其荷瘤体积反而较大[14]. 李素萍等采用了17 m/min,30 min/次的有氧运动干预对乳腺癌小鼠体内乳腺癌细胞的作用并不明显,而是通过促进小鼠对紫杉醇的吸收从而抑制肿瘤生长[22]. 而在几项运动联合药物干预研究中,其运动干预时间均为60 min,可抑制乳腺癌肿瘤的生长[15, 23]. 因此,我们将有氧运动方案设置为10 m/min,60 min/次,5次/周,共干预4周,结果能明显抑制乳腺癌小鼠肿瘤的生长.
瘤体积、瘤质量能够较好地反应4T1细胞原位移植乳腺癌模型小鼠肿瘤的生长情况. 至实验结束,TC组,TCE组平均瘤质量分别为1.69 g和0.98 g,显示有氧运动干预显著降低了瘤质量. 同时,根据最后瘤体积和生长曲线,中等强度有氧运动显著抑制了肿瘤的生长,且观察到TCE组小鼠精神状态明显好于TC组. 出于伦理道德和成本的考虑,本项实验有氧运动干预共进行了4周,未能进一步探讨各组小鼠最终存活时间,后续还需进一步实验.
现代研究发现炎症参与了恶性肿瘤的发生、发展和转移的全过程,被称为恶性肿瘤的第七大生物学特征[24]. 体内高水平的IL-1β被证实能够促进乳腺癌的发生发展[25]. 本项研究中,TC,TCE组相较于NC,NCE组,小鼠血清IL-1β质量浓度显著上升,说明接种4T1乳腺癌形成肿瘤的过程中出现了炎症反应,而有氧运动具有降低IL-1β的作用,减轻小鼠体内的炎症反应,改善肿瘤“微环境”,一定程度上可以遏制肿瘤细胞的恶性增殖.
内源、外源性雌激素质量浓度增多也会导致患乳腺癌风险增大,同乳腺癌相联系的各种危险因子均同雌激素有着一定关系且大多通过性激素有关路径产生影响. 雌二醇(E2)作为女性体内的重要性激素,病理条件下,将会导致乳腺癌细胞激增、转移、入侵等一系列行为的产生[26]. 人体和动物实验均显示,有氧运动干预可以降低E2质量浓度[27-28]. 本研究中,运动组相较于对照组,其E2质量浓度均出现了明显下降,表明无论是在正常小鼠或是乳腺癌模型小鼠中,有氧运动干预均可降低E2质量浓度,也支持了有氧运动可以降低患癌风险和抑制肿瘤生长的作用.
细胞凋亡是细胞基本生理活动之一,与肿瘤的发生和发展密切相关. 肿瘤的生长与细胞增殖速度和凋亡速度两方面相关,凋亡过程受抑则会导致细胞的过度生长,从而促进肿瘤细胞群体的生存和扩增. 因此,如何促进细胞凋亡途径诱导细胞死亡成为肿瘤研究的重要方面. NF-κB信号通路在肿瘤细胞抗凋亡中至关重要,抑制NF-κB信号通路过度激活可以调控凋亡蛋白的表达[29]. NF-κB是在B淋巴细胞中发现的一种转录因子,参与免疫与炎症反应相关的基因调控. NF-κB的过度激活可以促进肿瘤细胞增殖,抑制肿瘤细胞凋亡,促进肿瘤血管生成,提高肿瘤转移力,因此在肿瘤的发生发展中起着重要作用,其中P65∶P50 (NF-κB)组成的异二聚体起着主要的调控作用[30-31]. NF-κB过度激活可上调c-IAP,c-FLIP以及Bcl-2家族蛋白Bcl-x L等凋亡抑制因子的表达,从而拮抗细胞的凋亡. 同时,NF-κB可以激活具有抑制细胞死亡能力的基因的转录,表明了NF-κB与细胞的凋亡呈现敌对状态. 本研究中,模型组小鼠体内IL-1β质量浓度增加,出现了明显的炎症反应,受IL-1β等炎症因子刺激,抑制蛋白IκBα从在细胞质中的NF-κB与IκBα结合的三聚体复合物中解离出来,磷酸化并降解,从而释放NF-κBP65,使其得以转位进入细胞核中,与核内DNA上的特异序列相结合,从而启动或增强相关基因的转录[32-34].
-
中等强度有氧运动干预能够抑制4T1乳腺癌肿瘤的生长,其机制可能与有氧运动降低炎症因子IL-1β,减少IκBα蛋白的激活以降低NF-κBP65入核,抑制转录过程的发生,从而抑制NF-κB信号通路过度激活有关.







 DownLoad:
DownLoad: