-
衰老骨骼肌萎缩作为一种慢性衰老性疾病,其特征是骨骼肌质量及功能逐渐丧失,从而引发身体残疾、生活质量低下甚至死亡等不良健康事件的发生[1]. 就其发病机制而言,目前主要诱因有慢性疾病、炎症、内分泌紊乱、胰岛素抵抗、功能废用以及营养缺失等. 其中,蛋白质合成与降解失衡是导致其骨骼肌流失的重要因素[2],为此,增加骨骼肌蛋白合成,降低分解代谢则显得尤为重要[3]. 众所周知,运动作为有效干预策略之一,大量研究也已经证实其可以提高衰老骨骼肌细胞合成代谢水平而延缓肌萎缩. 然而,另一些研究却得出相反的结论,认为运动锻炼能改善衰老骨骼肌质量及力量,却无法提高蛋白合成[4]. 为此,可以推测骨骼肌蛋白降解可能在衰老骨骼肌萎缩发生的机制中扮演着更为重要的作用.
一般而言,骨骼肌内的蛋白降解主要通过两个途径,即细胞自噬和泛素-蛋白酶系统(Ubiquitin-proteasome system,UPS). 研究表明,运动训练可以通过抑制衰老骨骼肌UPS活性来减少肌肉降解和丢失[5],另有研究证实,在衰老小鼠模型中,运动可降低骨骼肌细胞自噬活性,缓解衰老导致的骨骼肌萎缩[6]. 然而,近期研究发现,长期高强度间歇训练可有效提高18月龄老年大鼠中骨骼肌自噬活性,抑制其骨骼肌萎缩[7]. 因此,细胞自噬与UPS二者相互作用协调维持着细胞平衡,从而调控骨骼肌质量. 目前研究多集中于衰老以后再进行运动干预,抗阻运动作为一种良好的锻炼方式,已经被证实可有效防止由于日常身体活动量不足而导致的肌肉量与肌力下降[8],而长期抗阻运动抑制衰老骨骼肌萎缩的作用机制还并未阐述清楚. 为此,本研究通过构建自然衰老小鼠骨骼肌萎缩模型,探讨长期抗阻运动对衰老骨骼肌细胞自噬及UPS二者之间的联系与机制,以期为衰老相关的骨骼肌萎缩防治提供重要的靶点与干预策略,进而为研究健康老龄化以及科学运动健身提供理论参考依据.
HTML
-
3月龄SPF级雄性小鼠18只,体质量46~55 g,由湖北省实验动物研究中心提供. 分笼饲养,自然光照,提供自由摄食和饮水,全程对动物的处置和操作符合动物伦理学要求.
-
RM2235转轮式切片机(德国徕卡),RT-6100型酶标仪(Rayto),垂直电泳仪及转移系统(Servicebio),6300化学发光仪(CLINX). 主要抗体包括抗Beclin1(CST,3498S),Bcl-2(CST,3498S),Atrogin-1(Abcam,ab168372),GAPDH(Proteintech,10494-1-AP) 以及羊抗兔IgG-HRP(CST,7074),多聚甲醛固定液(赛维尔生物),RIPA细胞裂解液(Servicebio),BCA蛋白浓度测定试剂盒(Servicebio)、蛋白上样Marker(Servicebio),PVDF膜(Servicebio),ECL化学发光超敏显色试剂盒(Servicebio).
-
随机分为青年对照组(YC)、老年对照组(OC)、长期抗阻运动组(LR),每组6只,LR组进行相应的长期运动干预.
-
本次实验采用中等强度尾部负重爬梯抗阻训练[9-10],运动干预时间在18:00-20:00. 爬梯高度1 m,爬梯台阶间隔2 cm,与地面成85°的倾角,小鼠放在爬梯底部,初始负重为小鼠自身质量的10%,刺激尾部诱导小鼠爬梯,适应训练1周后,增加自身质量的20%,然后每周增加体质量的20%,直至最终负重为小鼠体质量的80%,并持续训练14个月. 3次/周(隔天一次),2组/次,每组3次,组内间隔休息1 min,组间间隔休息2 min.
-
在运动干预结束后24 h进行采样,先完成小鼠体质量秤量后进行颈椎脱臼处死,迅速取左右后肢腓肠肌用生理盐水洗去血渍,滤纸吸除表面水分后称质量,置于冰上并记录对应质量,用于计算腓肠肌湿质量比. 随后,置于液氮中速冻,最后将样品及时转移-80 ℃冰箱保存备用.
-
腓肠肌湿质量比即通过把骨骼肌湿质量与受试体质量的比值(SI值)作为间接判断标准来比较骨骼肌萎缩的程度:SI=左右腓肠肌质量(mg)/体质量(g).
-
取合适大小腓肠肌放入离心管中经4%多聚甲醛固定(4 ℃过夜)、脱水、透明、浸腊、包埋制成石蜡标本,转轮切片机连续切片,厚度为5 μm,再经烤片、脱蜡、脱水后用苏木精和伊红分别对细胞核和细胞质染色,于400倍显微镜视野观察样本组织切片,采用Case Viewer软件对组织切片进行拍照,获取图像后使用Image-ProPlus 6. 0软件对每张图片进行分析,分别统计肌纤维的总面积及数量,从而得出单位肌纤维平均横截面积(μm2).
-
将样品腓肠肌组织于冰上加入细胞裂解液研磨后提取组织蛋白,离心获取上清后采用BCA法进行蛋白定量计算,再取5x蛋白上样缓冲液进行混匀,于100 ℃高温加热处理10 min后冷却放至冰箱备用. 电泳时,以上样量最低40 μg蛋白为标准来计算每组上样量. 根据目的蛋白的分子量选择合适浓度胶,常规上样、电泳、转膜. 转膜后将膜完全浸没于TBST稀释的5%的脱脂奶粉中,水平摇床室温孵育1.5 h封闭,用5% BSA-TBST稀释对应一抗,4 ℃水平放置摇床孵育过夜. 次日,使用TBST洗膜3次,每次5 min,加入二抗于室温摇床孵育1 h,再次TBST洗膜3次,每次5 min,最后ECL显影,化学发光仪成像. 获取图片后,选择Image J软件计算各个条带的灰度值,以GAPDH为内参蛋白,计算目的蛋白相对表达量,进而进行统计分析.
-
所有数据用平均数±标准差(X±SD)表示,采用Graphpad prism 9.0.0进行数据统计及图像生成,以单因素方差分析进行组间分析,p < 0.05,p < 0.01和p < 0.001分别为显著性差异、非常显著性差异、极其显著性差异.
1.1. 实验动物
1.2. 主要仪器设备与试剂
1.3. 实验分组
1.4. 运动方案
1.5. 取材
1.6. 骨骼肌湿质量比
1.7. HE染色
1.8. Western blotting检测
1.9. 统计方法
-
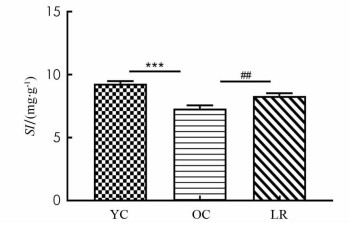
SI作为评估肌肉萎缩的间接有效指标,目前广泛应用于临床和基础实验[11-13]. 表 1及图 1结果显示,与YC组相比,OC组小鼠腓肠肌SI值显著降低(p < 0.001),然而,与OC组相比,LR组腓肠肌SI值均显著增加(p < 0.01). 同时显示,17月龄ICR小鼠发生了骨骼肌萎缩,长期抗阻运动能明显增加小鼠SI值而降低骨骼肌萎缩程度.
-
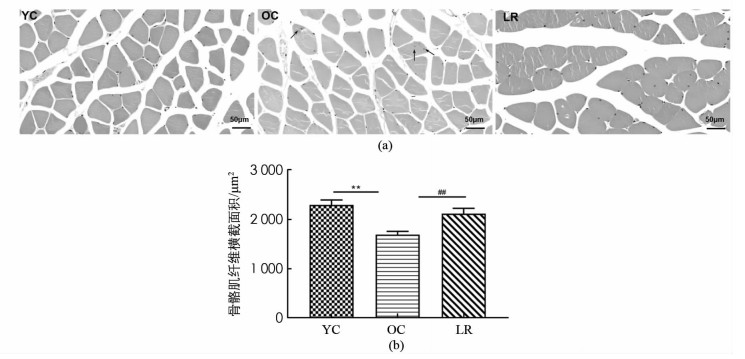
图 2结果显示,与YC组相比,OC组小鼠骨骼肌纤维横截面积显著降低(p < 0.01),并且出现肌纤维形状不规则及核内移现象,提示骨骼肌纤维衰老(图 2a),然而,与OC组相比,LR组骨骼肌纤维横截面积均显著增加(p < 0.01)如图 2(b). 表明17月龄ICR小鼠出现骨骼肌萎缩,长期抗阻运动增加了衰老小鼠骨骼肌纤维横截面积.
-
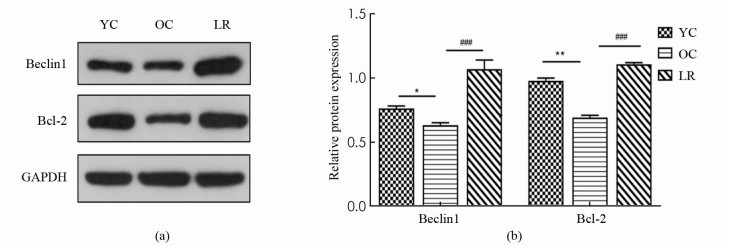
如图 3所示,与YC组相比,OC组小鼠骨骼肌Beclin1蛋白表达显著降低(p < 0.05),然而,与OC组相比,LR组小鼠骨骼肌Beclin1蛋白表达显著上升(p < 0.001),同时,与YC组相比,OC组小鼠骨骼肌Bcl-2蛋白表达显著降低(p < 0.01),然而,与OC组相比,LR组小鼠骨骼肌Bcl-2蛋白表达显著增加(p < 0.001).
-
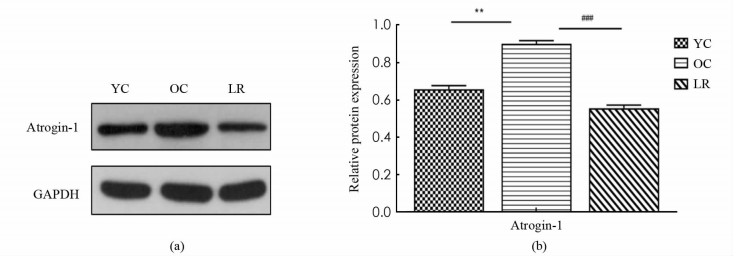
如图 4所示,与YC组相比,OC组小鼠骨骼肌E3泛素连接酶Atrogin-1表达显著增加(p < 0.01),与OC组相比,LR组小鼠骨骼肌Atrogin-1蛋白表达显著降低(p < 0.001).
2.1. 长期抗阻运动逆转了衰老小鼠骨骼肌SI值的下降
2.2. 长期抗阻运动增加衰老小鼠衰减的骨骼肌纤维横截面积
2.3. 长期抗阻运动激活衰老小鼠骨骼肌细胞自噬和增加细胞抗凋亡能力
2.4. 长期抗阻运动抑制衰老小鼠骨骼肌蛋白降解
-
ICR小鼠的生命周期比人类短得多,其50%存活年龄、平均存活年龄和最大存活年龄分别为20,18,31个月. 本实验运动干预开始时间为3月龄,动物处死时间17月龄,相当于小鼠平均寿命的65%以上[14]. 通过观察发现,衰老过程中小鼠出现如毛发灰暗脱落、眼睛局部受损等多种衰老体征,进一步证实17月龄小鼠达到衰老状态. 有研究观察到,8周抗阻运动训练显著提高了SAMP8快速老化小鼠腓肠肌SI值和肌纤维横截面积[15],本研究结果与上述一致,发现长期抗阻运动明显改善了17月老龄小鼠腓肠肌SI值,使肌纤维横截面积显著降低.
在饥饿、运动等应激条件下,细胞可形成双层或多层膜结构来吞噬自身胞质底物而形成自噬体,最后将底物运送到溶酶体与其结合形成自噬溶酶体,从而降解代谢废物,达到细胞自我更新的目的[16]. 其中,Beclin1作为第一个被确认的哺乳动物自噬调控基因,是自噬启动的重要生物标志[17]. 与此同时,衰老动物骨骼肌自噬水平低下,而运动干预则可以激活衰老骨骼肌细胞自噬活性,改善骨骼肌质量[18]. 并且,还有研究表明规律性运动可能通过激活自噬来减轻骨骼肌的衰老进程,起到延缓衰老的效果[19]. 本研究中,与青年对照组相比,老年对照组骨骼肌自噬蛋白Beclin1表达明显降低,而抗阻运动组中Beclin1表达显著上升,说明长期抗阻运动可激活衰老小鼠自噬活性,降解代谢废物以减少受损物质的累积,从而维持骨骼肌纤维内环境稳态.
细胞凋亡也调控和影响着细胞自噬活性,二者相互依赖,相互关联,从而维持机体分化、发育及体内平衡. 其中,Bcl-2作为同时调控细胞自噬与凋亡的重要基因,可通过抑制GSH外泄、Cyt-c释放进而阻止Caspase激活,起到抑制细胞凋亡的作用[20]. 与此同时,Bcl-2可以通过直接与Beclin1结合调控自噬启动,二者的相互作用可调节自噬与凋亡之间转换[17]. 已经有研究证实,8周中低强度抗阻运动增加了18月龄SD小鼠骨骼肌Bcl-2表达,减轻了细胞凋亡程度[21]. 本研究中,老年对照组与青年对照组相比,骨骼肌Bcl-2蛋白表达显著降低,而长期抗阻运动组显著逆转这一现象,增加Bcl-2蛋白表达,表明长期有效的运动干预增强了衰老骨骼肌细胞抗凋亡能力.
研究已经证实,Atrogin-1作为UPS中的关键E3泛素连接酶,在多种骨骼肌萎缩模型中特异性表达显著上调,是判断骨骼肌萎缩的一个关键因子[22]. 许多文献证实,运动训练能降低衰老骨骼肌中Atrogin-1的表达,近年来研究进一步发现,12周抗阻运动可抑制衰老大鼠骨骼肌Atrogin-1表达,改善其骨骼肌形态结构,有效缓解衰老骨骼肌萎缩[23]. 本研究中,长期抗阻运动中骨骼肌Atrogin-1蛋白表达的显著降低,进一步证实长期抗阻运动可有效抑制Atrogin-1介导的UPS活性,降低骨骼肌分解代谢,起到延缓衰老骨骼肌萎缩的作用.
综上,本研究表明,长期规律性抗阻运动可能通过激活细胞自噬活性,增加细胞抗凋亡能力并减少蛋白降解,从而抑制衰老骨骼肌萎缩.






 DownLoad:
DownLoad: