-
开放科学(资源服务)标识码(OSID):

-
我国是世界上最大的烟叶生产国,漂浮育苗是主要的育苗方式。然而,烟草苗期常出现多种病害,给烟叶生产带来严重的风险。迄今,苗期出现重大风险的病害有烟草立枯病、炭疽病、猝倒病、腐霉病、根腐病、靶斑病、灰霉病等[1]。2023年,贵州毕节烟草产区烟苗出现一未知病害,该病害初期发病症状与烟草立枯病高度相似,后期烟苗茎基部出现严重溢缩,根系断裂,造成烟苗成片枯萎死亡。为进一步明确发病原因并找到对应防控措施,有必要对其病际烟苗病原种类和病际微生态特征进行分析。
柯赫氏法则[2]是验证致病菌的经典方法,通过传统分离培养出病原菌,将病原菌进行回接,从而验证是否为致病菌。该方法只适用于病原菌为可培养致病菌的验证,但针对不可培养病原菌侵染所引起的病害,该法则并不适用。目前,基于高通量测序的扩增子测序技术是研究植物病害病际微生态的有效方法,可以了解病际真菌、细菌等微生物的菌群结构与多样性特征,从微生态学的角度揭示病害的潜在病原菌、致病过程[3],该技术在烟草病害上被广泛应用。已有研究表明,感赤星病烤后烟叶内生及叶际真菌群落多样性低于健康烟叶,感赤星病烤后烟叶链格孢属的相对丰度高于健康烟叶,前二优势属为链格孢属与红酵母属,与Alternaria alternata、A. longipes、A. tenuissim、A. yaliinficiens和A. tabacicola等多个链格孢属真菌导致的赤星病结果是一致的[4]。烟草感靶斑病烟叶亡革菌属(Thanatephorus)的相对丰度相对健康烟叶显著提升(71.84% vs 0.21%),而亡革菌属是靶斑病菌立枯丝核菌的有性型[5]。烟草亚隔孢叶斑病叶中亚隔孢壳属的丰度随病害严重程度的提高而提高[6]。感染白粉病的烟叶高氏白粉菌属(Golovinomyces)相对丰度相对健康烟叶提升显著(99.22% vs 91.86%)[7]。烟草细菌角斑病叶中假单胞菌属(Pseudomonas)的丰度相对健康烟叶提升显著(34.94% vs 8.88%)[8]。烤后霉变烟叶叶部和叶梗的曲霉属丰度较健康烟叶均提升显著(89.64% vs 40.13%、96.93% vs 62.77%)[9]。施用拮抗菌群后的烟草芽孢杆菌属(Bacillus)和寡养单胞菌属相比于对照分别增加3.9倍和7.02倍,其丰度与野火病病情指数呈显著负相关[10]。
针对贵州毕节烟草苗期出现的这一未知病害,本研究采用扩增子测序的方法分析了感病烟苗茎基部真菌和细菌的菌群结构和多样性特征,旨在探究其病害成因,为病害的防控技术研究提供参考依据。
HTML
-
2023年4月,贵州省毕节市威宁县暴发一未知苗期病害的烟草,在其苗床采集典型发病烟苗,主要表现为茎基部溢缩,后期病苗枯萎死亡(图 1)。采用五点取样法[11]分别随机选取5株感病和健康烟苗,使用75%酒精消毒的剪刀剪取烟苗茎基部的发病部位,同时采集健康烟苗相同部位的组织作为对照。感病组(WNB)5个样本的编号分别为WNB1、WNB2、WNB3、WNB4和WNB5,健康组(WNJ)5个样本的编号分别为WNJ1、WNJ2、WNJ3、WNJ4和WNJ5。
-
采用CTAB试剂盒[12]方法对基因组DNA进行提取。之后利用1%琼脂糖凝胶电泳检测DNA的纯度和浓度,取适量的样本DNA于离心管中,使用无菌水稀释样本至1 ng/μL。细菌使用515F和806R[13]进行扩增,真菌使用ITS5-1737F和ITS2-2043R[14]进行扩增。使用建库试剂盒进行文库构建,构建好的文库经过Qubit和q-PCR定量,文库构建合格后,使用Nova Seq 6000[15-17]进行PE250上机测序。
-
下机数据经过Cutadapt软件进行剔除以及过滤低质量序列,UCHIME算法去除嵌合体序列,最终得到有效序列。使用UPARSE软件对clean reads进行OTU聚类,筛选每个OTU的代表性序列,将OTU代表性序列与Unite数据库和Silva 132数据库比对,注释物种分类。利用R语言工具[18]绘制物种群落柱状图、主成分分析图和Venn分析图等。为了评估群落组成的复杂性并比较样本(组)之间的差异,在QIME2中,基于加权和非加权距离进行β多样性分析。在处理真细菌样本时,可以使用FunGuild工具,对主要的营养类型进行分类,研究具体的真菌与细菌功能分类,测序结果数据已上传NCBI数据库,真菌和细菌的序列登录号分别为PRJNA 1175465和PRJNA 1177533。
1.1. 样品采集
1.2. 病际真菌和细菌菌群结构与多样性分析
1.2.1. DNA提取及目标片段PCR扩增
1.2.2. 菌群结构分析
-
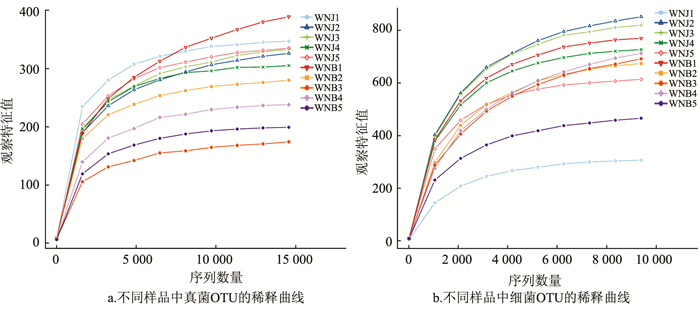
稀释曲线(Rarefaction Curve)结果表明,真菌样品随机抽取的测序数量达到15 000个时,曲线达到平台期(图 2a);细菌样品随机抽取的测序数量达到10 000个时,曲线达到平台期(图 2b),表明测序结果真实可靠具有代表性。
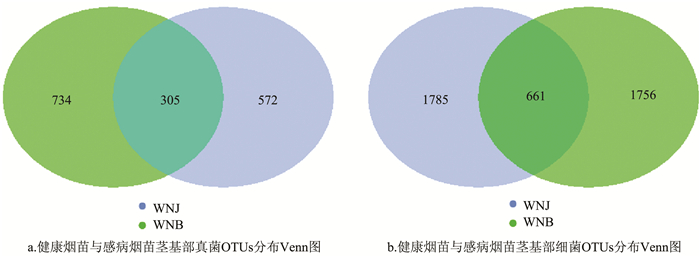
健康烟苗与感病烟苗的微生物群落差异分析Venn图(图 3)表明:在健康烟苗茎基部真菌群落中特有OTUs 572个,细菌群落中特有OTUs 1 785个;感病烟苗茎基部真菌群落中特有OTUs 734个,细菌群落中特有OTUs 1 756个。健康烟苗和感病烟苗茎基部真菌群落共有OTUs 305个,细菌群落共有OTUs 661个。
-
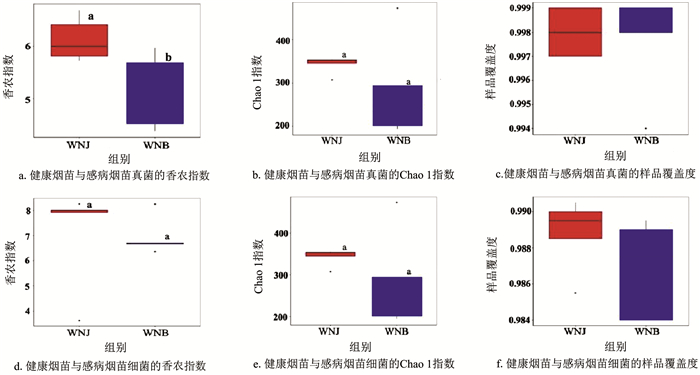
由图 4可知,测序的细菌与真菌样品组间的覆盖率均达到98.4%以上,表明测序结果真实合理。健康烟苗细菌的Shannon指数和Chao 1指数均高于感病烟苗,健康烟苗茎基部真菌Shannon指数显著高于感病烟苗(p<0.05)。
-
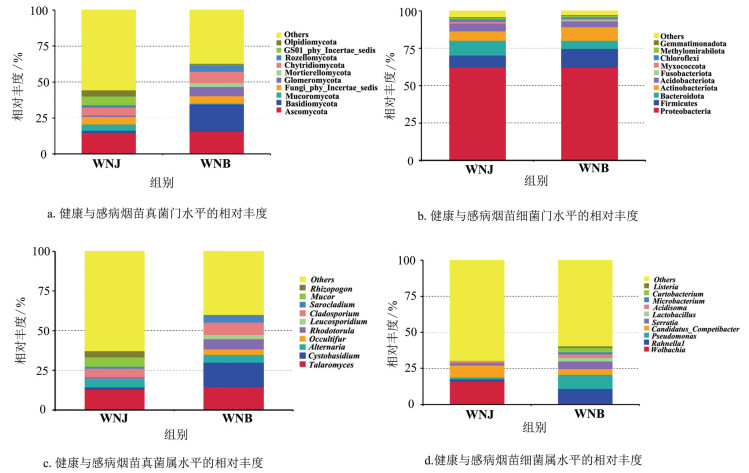
在真菌群落门水平上,感病烟苗与健康烟苗茎基部优势真菌门种类一致,其中子囊菌门(Ascomycota),相对丰度分别为47.49%与57.78%;担子菌门(Basidiomycota),相对丰度分别为38.57%与22.80%;毛霉门(Mucoromycota),相对丰度分别为1.52%与7.19%;球囊菌门(Glomeromycota),相对丰度分别为1.27%与2.14%(图 5a)。在细菌群落门水平上,感病烟苗与健康烟苗茎基部优势门水平种类一致,但其相对丰度具有差异性。其中,感病烟苗与健康烟苗茎基部均存在的优势细菌门为变形菌门(Proteobacteria,62.12%和62.40%)、厚壁菌门(Firmicutes,12.54%和7.68%)、拟杆菌门(Bacteroidota,5.40%和10.04%)与放线菌门(Actinobacteriota,9.23%和6.34%)。其中,健康烟苗变形菌门和拟杆菌门的相对丰度高于感病烟苗,感病烟苗厚壁菌门和放线菌门的相对丰度高于健康烟苗(图 5b)。
在真菌群落属水平上,感病烟苗与健康烟苗茎基部真菌的种类和相对丰度均有差异。其中,健康烟苗优势真菌属为蓝状菌属(Talaromyces,相对丰度为13.06%)、毛霉属(Mucor,相对丰度为6.00%)、枝孢菌属(Cladosporium,相对丰度为5.37%)、链格孢属(Alternaria,相对丰度为5.32%)和须腹菌属(Rhizopogon,相对丰度为3.85%)。感病烟苗优势真菌属为蓝状菌属(Talaromyces,相对丰度为14.58%)、囊胆菌属(Cystobasidium,相对丰度为15.25%)、枝孢菌属(Cladosporium,相对丰度为7.92%)、红酵母属(Rhodotorula,相对丰度为6.45%)和链格孢属(Alternaria,相对丰度为5.05%)(图 5c)。在细菌群落属水平上,感病烟苗与健康烟苗茎基部优势真菌种类与相对丰度均存在差异。健康烟苗优势细菌属为沃尔巴克氏体属(Wolbachia,相对丰度为16.29%)、肠球菌属(Enterococcus,相对丰度为2.60%)、沙雷氏菌属(Serratia,相对丰度为1.94%)、独岛杆菌属(Dokdonella,相对丰度为1.92%)和假单胞菌属(Pseudomonas,相对丰度为1.55%);感病烟苗优势细菌属为拉恩氏菌属(Rahnella,相对丰度为10.86%)、假单胞菌属(Pseudomonas,相对丰度为9.81%)、沙雷氏菌属(Serratia,相对丰度为5.27%)、短小杆菌属(Curtobacterium,相对丰度为2.97%)和酸球菌属(Acidisoma,相对丰度为2.45%)(图 5d)。
-
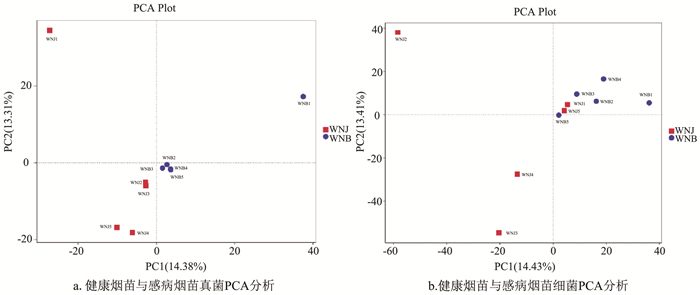
利用PCA图揭示了真菌和细菌群落的空间分布,样本距离越接近,表示物种组成结构越相似。因此,群落结构相似度高的样本倾向于聚集在一起,群落差异很大的样本则会距离较远。在真菌群落中,除WNB1外,其他4个感病样品倾向于聚集在一起,健康样品群落彼此分离(图 6a)。在细菌群落中,所有感病样品彼此聚集,健康样品彼此分离(图 6b)。分析表明,在真菌群落中感病组除了WNB1,其他样品彼此聚合,相似度较高,健康样本无论是真菌还是细菌都彼此分离,证明健康烟苗的各个样本相似度都不高。
-
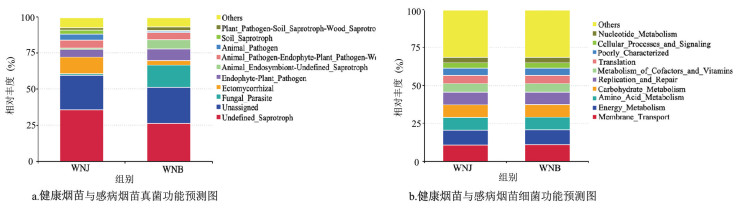
烟苗真菌群落功能预测柱形图(图 7a)表明,感病烟苗与健康烟苗茎基部真菌生态功能类群均主要分布于11个生态功能类群。健康烟苗茎基部真菌主要分布于外生菌根(11.44%)、内生植物病原菌(5.39%)和动物病原体-植物病原体-木材病原体-木腐生菌(5.32%),感病烟苗真菌主要分布于真菌寄生虫(15.35%)、内生植物病原体(7.97%)和未定义的动物内生菌-腐生菌(6.45%)。
烟苗细菌群落功能预测柱形图(图 7b)表明,健康烟苗和感病烟苗茎基部细菌生态功能类群相似,均主要分布于11个生态功能类群,相对丰度最高的均为膜运输(10.64%和11.01%),其次主要分布于能量代谢(9.82%和9.66%)、氨基酸代谢(8.40%和8.48%)、碳水化合物代谢(8.26%和8.40%)和复制与修复(8.41%和8.17%)。
2.1. 病际真菌和细菌菌群结构与多样性特征
2.1.1. 测序深度结果
2.1.2. 健康烟苗与感病烟苗真菌与细菌群落α多样性
2.1.3. 健康烟苗与感病烟苗真菌与细菌群落结构
2.1.4. 健康烟苗和感病烟苗真菌与细菌群落主成分分析
2.1.5. 健康烟苗和感病烟苗真菌与细菌功能预测
-
微生物群落结构改变、多样性改变、病原菌富集,是造成田间病害的主要原因。植株与微生物之间存在相互影响的关系,当部分微生物丰度增加时,植株就会表现出患病的状态,而当某一些病原物增加时,部分微生物也会产生增多或减少的变化,对微生物群落多样性产生影响[19]。张笑宇等[20]的研究发现,成熟期易感青枯烟田中可培养真菌数量显著高于健康烟田。孙美丽等[5]的研究发现,感烟草靶斑病的植株亡格菌属的丰度显著高于健康植株;郭涛等[21]的研究发现,假单胞菌在感中度野火病的病株中的丰度要高于轻度和高度感野火病的植株中的丰度。本研究发现,感病烟苗茎基部Talaromyces、Cystobasidium与假单胞菌的相对丰度相较于健康烟叶有增加的趋势,同时感病烟苗真菌和细菌的多样性相较于健康烟苗都有降低的趋势,并且感病烟苗真菌的shannon指数与健康烟苗存在显著差异,这与刘亭亭等[22]的研究结果一致,进一步证实了真菌性病害发生时病际组织的真菌多样性低于健康组织这一普遍规律。
本研究中,感病烟苗中Talaromyces与Cystobasidium含量相较于健康烟苗有所增加。虽然Talaromyces是一种研究较少的病原菌,但仍有研究表明Talaromyces可引起多种植物病害。Stošić等[23-24]发现,T. minioluteus与T. rugulosus可引起梨、洋葱、番茄和橙子的腐烂;Liu等[25]的研究发现,T. funiculosus可引起玉米穗腐病,从而降低玉米品质与产量。感病样本中拉恩氏菌属(Rahnella)、假单胞菌(Pseudomonas)、Cystobasidium含量显著增加。据报道,Rahnella aquatilis会导致鲫鱼病害[26],Rahnella sp. 会引起伊朗黑杨溃疡病[27],假单孢属能引起烟草细菌性角斑病、野火病[5, 10]。目前尚未有Cystobasidium致病的报道。考虑到以往研究中病害组织相对健康组织丰度显著提高的属均有病原菌种被报道,因此,下一步需要选择性分离拉恩氏菌属、假单胞菌、Cystobasidium和蓝状菌属,并遵循科赫氏法则进行回接试验以确认病原菌。
感病烟苗相对于健康烟苗的假单胞菌丰度呈增加趋势。有研究报道,假单胞菌既可作为病原菌危害植物健康,如烟草野火病[28],也可作为有益菌诱导寄主植株产生抗性[29]。假单胞菌在未知真菌侵染植物的过程中所起的作用还有待下一步验证。






 DownLoad:
DownLoad: