-
开放科学(资源服务)标识码(OSID):

毛果杜英(Elaeocarpus apiculatus)和水石榕(E. hainanensis),属杜英科(Elaeocarpaceae)杜英属(Elaeocarpus),分布于我国海南、云南和广东,是热带、亚热带常绿阔叶林森林群落的重要组成树种,也是园林绿化的优良树种之一,在广东地区的城市道路、公园绿地、森林公园以及生态公益林等地大量栽植[1-3]。然而,2020—2022年,广州、深圳、东莞等地城市绿地的杜英频发一种疑似致死性树皮溃疡病,表现为树皮肿胀、开裂、凹陷,枝干局部或整株枯死,恶性程度高,初期常被误判为枝枯病[4]。随着病菌侵染加重而导致整株树木枯死,广州市部分地区的感染率达到80% 以上,枯死率达到15.38% ~ 62.50%[5],给城市林业和园林造成管养困难和较大的经济损失。2022年,Huang等[5]通过形态特征及ITS、LSU、tef1、rpb2等多基因系统发育分析,确认杜英疫病病原菌属于新属新种,命名为杜英生假隐丛赤壳菌(Pseudocryphonectria elaeocarpicola)。该菌隶属于子囊菌门(Ascomycota)粪壳菌纲(Sordariomycetes)粪壳菌亚纲(Sordariomycetidae)间座壳菌目(Diaporthales)隐丛赤壳科(Cryphonectriaceae)。隐丛赤壳科包含多种引起木本植物枯萎或溃疡的病原真菌[6],例如板栗疫病(Cryphonectria parasitica)、桉树溃疡病(Celoporthe eucalypt)[7]等。杜英属植物上已报道Amphilogia gyrosa会引起新西兰齿叶杜英(Elaeocarpus dentatus)树皮溃疡病[8]以及隐丛赤壳科的新成员杜英生假隐丛赤壳菌引起的杜英疫病[5]。
对杜英生假隐丛赤壳菌的研究,仍处于起步阶段,主要集中在生物学特性和致病性测定、全基因组测序和早期快速诊断等方面。官莉莉等[9]采用生长速率法,测定了杜英疫病病原菌最适生长碳源、氮源、温度、pH值等生物学特性,并通过烫伤接种的方法探究致病性。结果表明,杜英疫病病原菌最适生长碳源为可溶性淀粉、乳糖和纤维素粉,氮源为蛋白胨和酵母浸粉,pH值为4.0,最适生长温度为30 ℃,接种结果发现对山杜英(Elaeocarpus sylvestris)也具有致病性。Yang等[10]利用DNBSEQ和PacBio平台对杜英生假隐丛赤壳菌进行了全基因组测序,预测该菌致病过程关键毒力因子,这对于研究致病机制和制定更有效的防治策略是必要的。针对早期病症不明显问题,黄灿等[11]建立了杜英疫病菌RAA-CRISPR/Cas12a-LFD检测体系,具有反应温度易达到、高特异性、高灵敏度和易操作等优点,适合在野外或缺乏实验室检测设备的场景下对杜英疫病菌的检测,为该病害的早期监测和预警提供了重要技术支持。目前还未见开展杜英疫病化学防治药剂筛选的报道。本研究通过田间采样、分离纯化、回接致病,并采用菌丝生长速率法,评估了多种杀菌剂对杜英生假隐丛赤壳菌的毒力,旨在揭示杜英生假隐丛赤壳菌药剂敏感谱,筛选出兼具效果和环境安全性的防治药剂,为后期制定科学、绿色、可持续的防控策略提供依据。
HTML
-
病样采集于广州市南沙区黄山鲁森林公园(22.78°N,113.56°E)发病的毛果杜英活立木的茎基部,将其带回实验室分离。切取病健组织交界处的树皮样品(约5 mm×5 mm×2 mm),依次用无菌水清洗样品表面杂质,2%次氯酸钠处理1 min,70%酒精处理1 min,最后用无菌水清洗3次,并在滤纸上滤干;将清洗好的样品用滤纸吸去多余的水分,置于马铃薯葡萄糖琼脂(PDA)平板上,在30 ℃、黑暗条件下培养;2 d后出现目标菌落,挑取少量菌丝转接到新的PDA平板上再培养7 d,得到真菌分离物;进一步进行单孢分离,得到分离物纯培养NSHLS-DY-1。
-
在接种之前,取菌块接种在PDA上,置于30 ℃条件下培养7 d得到新鲜的菌板;具体的接种方法参考Chen等[12]的方法稍作修改:利用5 mm打孔器在毛果杜英苗(胸径5 mm的1年生苗)茎基部约20 cm处打孔,裸露树干形成层,然后用匹配对应的打孔器取得新鲜病原菌菌落,将有菌落一面覆盖在树干形成层处;无菌的PDA平板作为对照;最后用parafilm封口膜封口,设置3次重复,并持续观察毛果杜英发病情况;遵循柯赫氏法则,从病变中的组织重新分离,并通过形态学确认病原菌的致病特性。
-
将病原菌菌块置于PDA培养基上,在30 ℃条件下培养,直至长出子实体结构。为了进一步观察和测量菌株的分生孢子梗、产孢结构和分生孢子形态,将子实体压碎于含无菌水的显微镜载玻片上,用100倍油镜在莱卡倒置荧光显微镜(DMI8,徕卡/Leica)下观察并拍照。
-
参照吴发红等[13]的方法,利用SDS法提取菌株基因组DNA;以提取的基因组DNA为模板,采用通用引物ITS4和ITS5[14]进行PCR扩增,引物由北京擎科新业生物技术有限公司合成。50 μL反应体系:10 mmol/L dNTP Mix 1 μL、Phanta Max Super-Fidelity DNA Polymerase 1 μL、5 μmol/L正反向引物各1 μL、2×Phanta Max Buffer 25 μL、DNA模板(10 ng)1 μL,ddH2O补足至50 μL。PCR反应程序:95 ℃预变性3 min;95 ℃变性15 s,55 ℃退火30 s,72 ℃延伸1 min,共34个循环;最后72 ℃延伸5 min。扩增产物用2%琼脂凝胶电泳检测DNA片段,其中rDNA ITS扩增目的片段约600 bp。将有目的片段的PCR产物送至北京擎科新业生物技术有限公司测序,将测序所得序列在GenBank中进行BLAST比对,利用Mega 7.0软件以Clustal X方法进行多序列比对,以邻接法构建系统发育树,确定菌株的分类地位。
-
采用菌丝生长速率法测定8种杀菌剂对病原菌的抑制作用。将8种供试农药配制成2 000 μg/mL的母液,加入无菌水进行系列稀释,通过预试验得到供试药剂对不同病原菌的有效浓度范围。咪鲜胺、戊唑醇、咯菌腈、丙环唑和苯醚甲环唑系列浓度为0.1、0.05、0.025、0.012 5、0.006 25 μg/mL;嘧菌酯系列浓度为10、5、2.5、1.25、0.625 μg/mL;多菌灵系列浓度为10、5、2.5、1.25、0.625、0.312 5 μg/mL;啶酰菌胺系列浓度为1 000、800、400、200、100、50 μg/mL(表 1)。制备含药PDA培养基,倒平板,备用。将直径5 mm的菌饼接种到各含药和空白对照平板上,置于30 ℃条件下培养,设置3次重复。培养6 d后,对照组病原菌菌落接近长满平板,采用十字交叉法分别测量各处理的菌落直径。
计算各处理浓度的抑制率:抑制率=(对照菌落增长直径-处理菌落增长直径)/对照菌落增长直径×100%。采用SPSS软件Probit求出毒力回归方程y= ax+b、相关系数r和药剂抑制中浓度EC50,横坐标为药剂浓度的对数,纵坐标为反应抑菌率值,评估筛选出的杀菌剂对病原菌的抑菌能力。
1.1. 病原菌的分离与纯化
1.2. 分离菌株的致病性测定
1.3. 病原菌鉴定
1.3.1. 形态鉴定
1.3.2. 分子鉴定
1.4. 药剂室内毒力测定
-
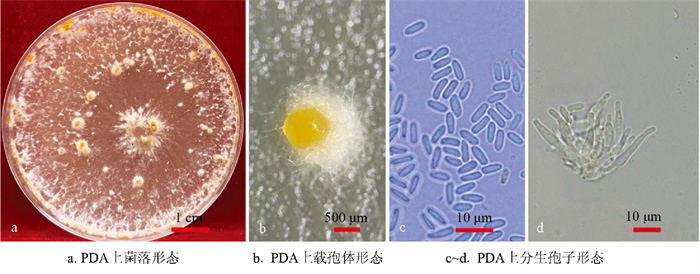
将病样分离菌株在毛果杜英幼苗上接种14 d后,由图 1可知,毛果杜英幼苗整株致死,在幼苗接种点均引起溃疡病斑(图 1c),病斑周围产生子实体结构分生孢子角(图 1d);而接种PDA培养基的空白对照的幼苗接种点上未见病斑,未出现毛果杜英幼苗致死的情况。可见,毛果杜英幼苗接种后的发病症状,其特征与林间危害状相似(图 1a),与产生的子实体结构也相似(图 1b);另外,接种后再分离的培养物与接种菌落形状一致,而空白对照未分离到隐丛赤壳科病原菌,验证了柯赫氏法则。因此,菌株NSHLS-DY-1为杜英溃疡病病原菌的致病菌。
-
菌株NSHLS-DY-1在PDA培养基上的菌落初期为白色,培养10 d后逐渐变为黄白色(图 2a),形成载孢体,其初期为橙黄色(图 2b),后期逐渐变为暗黄色;分生孢子透明,无隔,纺锤形,常呈瓜子状(图 2c),产孢细胞为瓶梗状(图 2d),圆柱形,有或无瘦长顶点。根据菌株NSHLS-DY-1的形态特征,上述形态特征与文献中对隐丛赤壳科[5-6]的描述一致。
-
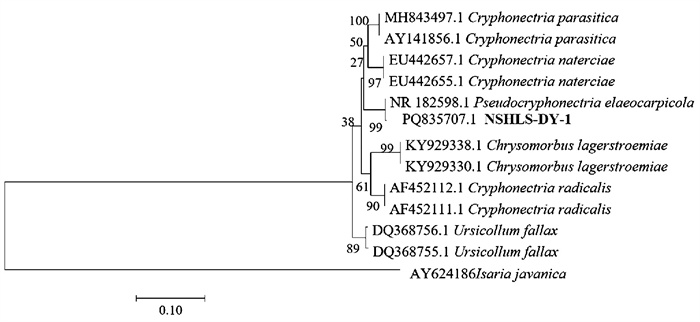
BLAST比对结果表明,菌株NSHLS-DY-1的rDNA ITS序列与杜英生假隐丛赤壳菌的相似性均达99%。进一步构建系统发育树,发现菌株NSHLS-DY-1与杜英生假隐丛赤壳菌(NCBI序列登录号为NR182598.1)聚在同一分支上(图 3)。因此,结合形态学特征和分子鉴定结果,确定菌株NSHLS-DY-1为杜英生假隐丛赤壳菌(Pseudocryphonectria elaeocarpicola)。
-
采用菌丝生长速率法测定了8种杀菌剂对尖叶杜英树皮溃疡病菌的毒力,由表 2可知,病原菌对50%多菌灵水分散粒剂WG表现最敏感,其EC50最小,为0.015 μg/mL,其次是15.6%丙环唑,EC50为0.022 μg/mL;另外,对2.5%咯菌腈、43%戊唑醇、45%咪鲜胺、10%苯醚甲环唑也表现较敏感,其EC50分别为0.030、0.044、0.053、0.092μg/mL;而对啶酰菌胺的敏感性表现最差,其EC50为592.650 μg/mL。因此,综合得出多菌灵、丙环唑、咯菌腈、戊唑醇、咪鲜胺、苯醚甲环唑对尖叶杜英树皮溃疡病菌有较强的毒力。
2.1. 杜英溃疡病病原菌的致病性
2.2. 杜英溃疡病病原菌的鉴定
2.2.1. 形态鉴定
2.2.2. 分子鉴定
2.3. 不同药剂对杜英生假隐丛赤壳菌室内毒力测定
-
杜英(Elaeocarpus spp.)不仅是华南地区山地次生林中的建群优势树种,在区域乡土植物体系中占据重要生态位,同时在城市绿化和景观建设中被广泛应用,具有较高的生态与园艺价值[15]。近年来,在广州等地陆续发现毛果杜英与水石榕发生严重的枝干溃疡病害,呈现扩展性强、危害性大的特点,需引起重视。本研究从一株毛果杜英病株中分离获得的菌株NSHLS-DY-1,依据柯赫氏法则,通过回接试验证实其为致病因子,并经形态观察及ITS序列比对,明确其为杜英生假隐丛赤壳菌,与Huang等[5]的研究结果一致。
杜英生假隐丛赤壳菌隶属隐丛赤壳科(Cryphonectriaceae),该科为重要的林木植物病原真菌,Gryzenhout等[16]和Suzuki等[17]指出其寄主极广,已知影响超过14科100余种木本植物。我国目前已报道的该科病原菌还包括隐丛赤壳属(Cryphonectria)、黄隐丛赤壳属(Chrysoporthe)、暗隐丛赤壳属(Celoporthe)、浅隐丛赤壳属(Corticimorbus)、金隐丛赤壳属(Chrysomorbus)等[18],其中杜英生假隐丛赤壳菌是其中新近报道的成员之一[5]。最被人们所熟知的栗疫病病原菌寄生隐丛赤壳菌,是我国林业检疫性有害病原菌,在全世界广泛危害栗属植物,导致果实产量和质量下降,严重时导致植株整株死亡,20世纪初期该病害的暴发曾导致美洲及欧洲栗树大规模减产,北美栗树种植产业濒临崩溃。
针对隐丛赤壳科化学药剂敏感性方面的研究,目前在栗疫病隐丛赤壳菌上研究较多。已用于防控栗疫病的化学药剂,包括甲基托布津、氯化铜、多菌灵、丙环唑以及咪鲜胺等[19]。González-Varela等[20]测试了6种农药[克菌丹、丙环唑、嘧菌酯、福美双酰胺、多菌灵+氟环唑、氟硅唑(三唑类DMI杀菌剂) + 多菌灵]对栗疫病的抑菌效果,发现丙环唑是最有效的杀菌剂,即使在最低浓度下也能抑制真菌生长。Trapiello等[21]进一步证实,丙环唑等三唑类对栗疫病的控制效果最佳。本研究参考了部分板栗疫病隐丛赤壳菌常用化学药剂,评估了8种常用杀菌剂对杜英生假隐丛赤壳菌的体外毒力,发现50%多菌灵、15.6%丙环唑、2.5%咯菌腈、43%戊唑醇、10%苯醚甲环唑等药剂对病原菌具有显著抑制作用,均表现出良好毒力水平,可见,研究结果与栗疫病隐丛赤壳菌药剂敏感谱较为一致,对多菌灵和三唑类药剂表现较佳的药物敏感性。相关研究表明,这些药剂主要通过干扰真菌细胞膜合成、影响有丝分裂微管形成或阻断能量代谢通路等发挥作用[22-25]。
嘧菌酯、吡唑醚菌酯和啶酰菌胺是目前广泛应用于植物病害防治的主要真菌呼吸抑制剂,属于SDHI类杀菌剂,是防控多种植物病原真菌的重要手段[26]。它们通过结合在线粒体复合物II的泛醌结合位点(Q位点),阻断病原菌呼吸作用[27]。新一代SDHI杀菌剂(如啶酰菌胺、吡唑醚菌酯、氟啶胺等)具有更广谱的杀菌活性。本研究结果发现,相比之下,啶酰菌胺、嘧菌酯对杜英生假隐丛赤壳菌的抑菌效果较差,可能由于SDHI属于位点特异性杀菌剂[28],对杜英生假隐丛赤壳菌的抑菌防治效果较差。因此,在杜英疫病城市园林管理实践中,嘧菌酯、啶酰菌胺不作为杜英疫病的首要推荐用药。
Cheradil等[29]测试了4种杀菌剂(二氟菌脲+啶酰菌胺、嘧菌酯+苯醚甲环唑、Score 250 EC-苯醚甲环唑和三碱式硫酸铜)在体外对栗疫病的毒力,发现Score 250 EC-苯醚甲环唑和嘧菌酯+苯醚甲环唑对真菌菌丝生长的抑制作用最强。Derelli等[30]的研究发现吡唑醚菌酯与啶酰菌胺的复配制剂能有效抑制栗疫病的生长。可见,将三唑类与SDHI类杀菌剂如啶酰菌胺、嘧菌酯复配能有效提高隐丛赤壳菌的药物敏感性。在后期研究中,将进一步开展三唑类与SDHI类杀菌剂复配对杜英疫病的防治效果。另外,本研究是基于体外菌丝生长抑制,缺乏田间防效验证,后续将进一步开展系统药效实验,明确施药浓度、频次和配方组合,并进一步评估长期施药后病原菌的抗药性发展风险。综上所述,杜英生假隐丛赤壳菌已成为影响华南地区杜英健康的重要病原,其潜在危害性不容忽视。本研究为病原识别与药剂选择提供了理论依据,并为后续田间防治与生态安全评估奠定了基础。






 DownLoad:
DownLoad: