-
开放科学(资源服务)标志码(OSID):

-
旁粒是在哺乳动物染色质区域新发现的一类核内亚细胞器,大多分布在核散斑附近的染色质间区,由属于非编码RNA的NEAT1(Nuclear Enriched Autosomal Transcript 1)和DBHS (Drosophila Behavior Human Splicing)蛋白家族中的P54NRB/NONO (on-POU domain-containing octamer-binding protein)、PSPC1(Paraspeckle component 1)、SFPQ/PSF(PTB-associated splicing factor)相互作用共同形成[1-2].NEAT1是旁粒的基本组成结构,可以与NONO和PSPC1直接结合,并促使它们定位在旁粒上[3].
旁粒能够参与DNA损伤修复、转录调控、细胞增殖等一系列细胞生长活动,其在细胞生长过程中发挥着重要作用. 细胞在生长发育的过程中会不断面临外源性或内源性应激,可能会诱发DNA损伤最终导致基因组不稳定和细胞死亡[4]. 为了维持稳定,细胞进化出了复杂且相互作用的DNA损伤反应(DNA Damage Repair,DDR)应答网络. 研究表明,敲低NONO基因表达会引起DNA双链断裂(Double Strand Breaks,DSB)修复的延迟,NONO有助于快速准确地修复人体细胞内产生的DSB[5]. Gao等[6]发现敲低PSPC1会使G2/M期细胞增多,抑制细胞增殖;在HeLa细胞中敲低PSPC1会导致细胞中γH2AX蛋白水平增加,过表达PSPC1抑制γH2AX蛋白水平增加,由此推测PSPC1可能参与DDR. 另有研究发现,SFPQ和NONO形成复合物共同促进DNA底物序列独立配对,有助于在复杂的细胞环境中进行DNA损伤修复[7];并且能够促进γH2AX人胰岛素生长因子结合蛋白3(IGFBP-3)中的DSB修复[8];NONO和SFPQ还可以作为一种与RNA∶DNA混合相关的新型端粒稳定调节剂,抑制端粒的退化和同源重组,确保端粒的完整性[9]. NONO,PSPC1与细胞的增殖也息息相关. 在甲基甲烷硫酸盐(MMS)引起的DDR中,敲低PSPC1会导致HeLa细胞凋亡水平显著增加,使大量细胞进入G2/M期,而过表达PSPC1细胞则会减少细胞凋亡数量,使得更多细胞在G1/S期积累[10]. NONO既能促进细胞增殖,也能抑制细胞增殖. 在食管鳞状细胞癌(ESCC)细胞模型中,NONO沉默可以显著抑制ESCC增殖[11];在HeLa细胞中,缺少NONO会降低细胞生长速度[12],表明NONO的表达与细胞增殖呈正相关. 在体外,NONO被敲低会引起小鼠心脏成纤维细胞过度增殖[13];在人外周血的单核细胞(THP1)中,抑制NONO表达会促进THP1细胞增殖[14];在结肠直肠癌(CRC)细胞中,胃腺癌预测基因(GAPLINC)的表达会促进CRC细胞增殖,SFPQ和NONO被发现可以结合GAPLINC最终抑制细胞增殖[15].
目前,旁粒相关研究多集中在人和小鼠,对其在其他物种上的作用尚不清楚. 本研究首先克隆水牛NONO,PSPC1编码区序列,分析水牛胎儿成纤维细胞中旁粒相关基因的表达水平;构建细胞衰老模型,通过上调NONO,PSPC1表达,阐述NONO,PSPC1在水牛细胞增殖及抗衰老方面的作用.
HTML
-
水牛胎儿来自广西南宁屠宰场,取耳部部分皮肤组织用于细胞原代分离培养,肌肉、心、肝、脾、肺、肾、胃、卵巢和睾丸组织浸润液氮后,储存到-80 ℃用于RNA提取.
-
pEGFP-N1质粒由本实验室提供.
-
胎牛血清、高糖DMEM粉末、0.25%EDTA胰蛋白酶、青链霉素来自GIBCO公司;二甲基亚砜(DMSO)、各类无机盐药品、抗生素氨苄(Amp)来自Sigma公司;Trizol Reagent来自Thermo FIsher公司;DL5000 DNA Marker、DNA Marker Ⅶ、Supercoiled DNA Ladder Marker、T4 DNA连接酶、限制性内切酶来自TaKaRa公司;Mix(green)Golden Star T6 Super PCR Mix来自北京擎科新业生物公司;HiScript © Ⅲ RT SuperMix for qPCR、qRT-PCR荧光染料(2×ChamQ Universal SYBR qPCR Master Mix)来自南京诺唯赞生物科技股份有限公司.
-
根据美国国家生物技术中心(NCBI)公布预测的水牛NONO(XM_006079429.2)和PSPC1 (XM_025262782.1)基因mRNA序列,应用Oligo 7.0软件设计适用于基因克隆和qRT-PCR的特异性引物,送至上海生工公司合成. 基因克隆引物在NONO,PSPC1的上下游分别引入XhoⅠ,PstⅠ和XhoⅠ,KpnⅠ酶切位点序列,引物序列见表 1. 水牛SFPQ(XM_025289121.1)和β-actin的qRT-PCR特异性引物设计同上,qRT-PCR引物亦见表 1.
-
参考邢青华等[16]的方法进行水牛胎儿成纤维细胞分离培养.
-
将PCR产物目的片段进行胶回收,并将目的片段连接至pEASY-Blunt-Simple克隆载体,挑选阳性质粒进行测序.
-
利用DNA胶回收试剂盒将线性载体片段和目的片段回收,进行连接反应,构建pEGFP-N1-NONO和pEGFP-N1-PSPC1真核表达载体,分别用XhoⅠ,PstⅠ和XhoⅠ,KpnⅠ进行双酶切鉴定. 将TurboFect转染试剂(μL)与质粒质量(ng)按照4∶1的比例,在200 μL DMEM培养液(无双抗、无血清)中加入4 μL转染试剂和1 ng质粒,孵育15~20 min后加入细胞培养皿中.
-
将细胞以104个/mL,每孔100 μL细胞培养液,接种于96孔板中,每个试验组设置3~5个重复,同时设置空对照组,放置到细胞恒温培养箱中培养. 24 h后,避光操作,每孔加入10 μL CCK-8试剂. 避光孵育1.5 h后,酶标仪设置450 nm吸光度后进行检测记录. 每2 d换1次细胞培养液,保持每次处理时间相同、孵育时间相同. 连续7~8 d统计数据,以培养天数为横坐标,450 nm吸光度为纵坐标绘制细胞生长曲线.
-
收集P0,P5,P10,P15代细胞,利用TRIzol试剂提取细胞总RNA,利用诺唯赞HiScript © Ⅲ RT SuperMix for qPCR试剂反转录成cDNA. 以稀释的cDNA为模板进行实时荧光定量PCR扩增,反应体系为2×ChamQ Universal SYBR qPCR Master Mix 10 μL,上下游引物各0.4 μL,cDNA 1 μL,ddH2O 8.2 μL. 反应程序:预变性95 ℃ 2 min;变性95 ℃ 10 s,退火60 ℃ 30 s,延伸37 ℃ 30 s,40个循环;熔解曲线95 ℃ 15 s,60 ℃ 30 s,95 ℃ 15 s. 样本重复3次,使用2-ΔΔCt方法分析结果.
-
弃除细胞培养液,用PBS缓冲液清洗细胞1~2遍,加入β-半乳然后糖苷酶染色固定液1 mL,室温固定15 min. 弃固定液,PBS缓冲液洗涤细胞3次,每次3 min. 加入用聚丙烯容器配置好的1 mL β-半乳糖苷酶染色工作液(A液10 μL,B液10 μL,C液930 μL,X-Gal液50 μL). 避光37℃孵育8 h,普通光学显微镜下观察计数. 显微镜下每皿随机抽取5个视野,记录阳性细胞与总细胞个数,5个视野的总阳性细胞数与总细胞数的比值为β-Gal细胞阳性率.
-
待细胞汇合度在80 %左右时,用4 %多聚甲醛室温固定30 min,0.5 %Triton X-100透化30 min,5% BSA封闭液封闭30 min,再用1% BSA分别稀释抗体,一抗4 ℃冰箱孵育8 h(其中阴性对照孔不需加入一抗,只加1% BSA). 第2 d用二抗室温避光孵育2 h,Hoechst染色15 min,染色结束加入适量PBS,荧光显微镜拍照记录,每组试验重复3次. 试验过程中每次处理之后都用PBS清洗细胞3次.
-
实验数据显著性差异分析参考统计软件SPSS 19.0. 基因序列参考NCBI已公布的不同物种的NONO和PSPC1基因序列,利用SnapGene软件对测序结果进行比对,并根据MegAlign软件进行同源性比对和同源进化树构建.
1.1. 材料与试剂
1.1.1. 样品
1.1.2. 质粒
1.1.3. 试剂
1.1.4. 引物设计与合成
1.2. 方法
1.2.1. 细胞培养
1.2.2. 克隆与测序
1.2.3. 重组质粒构建及细胞转染
1.2.4. 生长曲线测定
1.2.5. qRT-PCR检测
1.2.6. β-半乳糖苷酶染色
1.2.7. 免疫荧光
1.2.8. 数据统计与分析
-
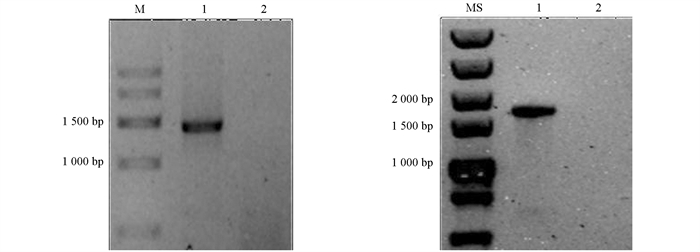
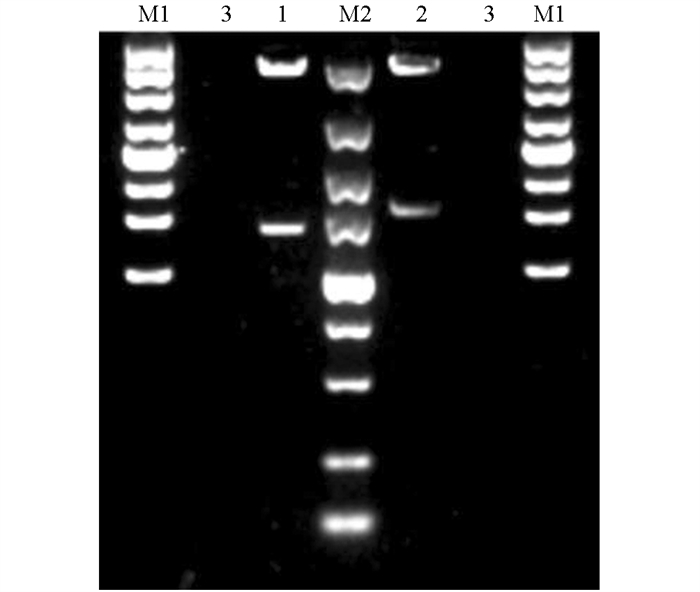
采用广西本地水牛卵巢组织提取的总RNA进行反转录,以cDNA为模板进行PCR,分别扩增出大小约为1 500 bp的NONO(图 1a)和PSPC1特异性条带(图 1b). 将目的片段连接至pEASY-Blunt-Simple克隆载体,挑选阳性质粒进行测序. 结果分别得到1 424 bp和1 574 bp的水牛NONO和PSPC1基因编码区序列,与Genbank预测序列(NONO:XM_006079429.2;PSPC1:XM_025262782.1)一致. 将NONO和PSPC1基因编码区序列连接到pEGFP-N1载体中,并分别用XhoⅠ,PstⅠ和XhoⅠ,KpnⅠ对pEGFP-N1-NONO和pEGFP-N1-PSPC1进行双酶切鉴定,结果显示酶切片段大小与预期一致(图 2).
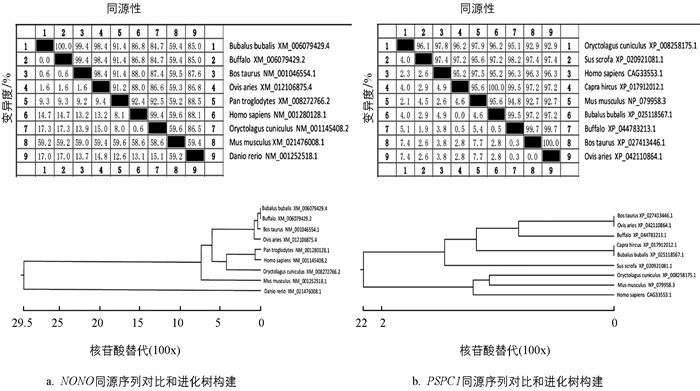
采用Jotun Hein法进行同源性比对和进化树构建(图 3). 结果发现,NONO基因在本地水牛与印度水牛、牛、绵羊、人、小家鼠、黑猩猩、斑马鱼氨基酸序列相似性分别为99.9%,98.9%,95.0%,91.6%,95%,94.5%和65.3%. PSPC1基因在本地水牛与印度水牛、牛、绵羊、山羊、人、野猪、小家鼠、穴兔氨基酸序列相似性分别为99.7%,99.5%,86.0%,98.8%,89.3%,70.9%,70.1%和89.9%.
-
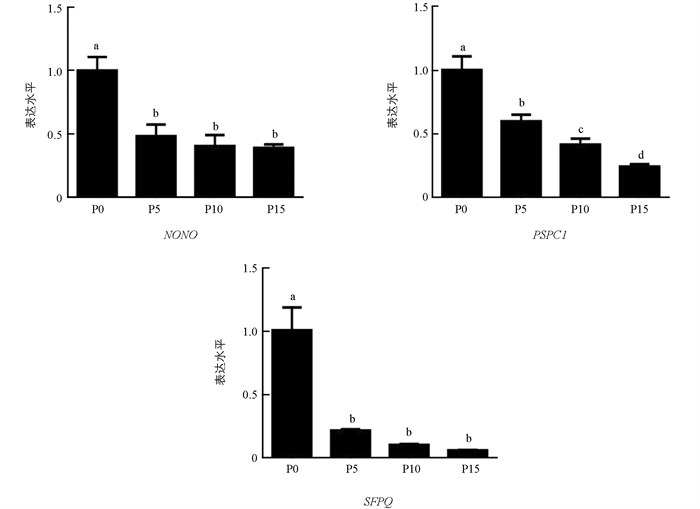
qRT-PCR结果显示,随着细胞培养代数增加,PSPC1表达显著降低(p < 0.05);NONO和SFPQ表达水平整体呈现出下降趋势,在5,10,15代细胞中的表达差异不具有统计学意义(p > 0.05)(图 4).
-
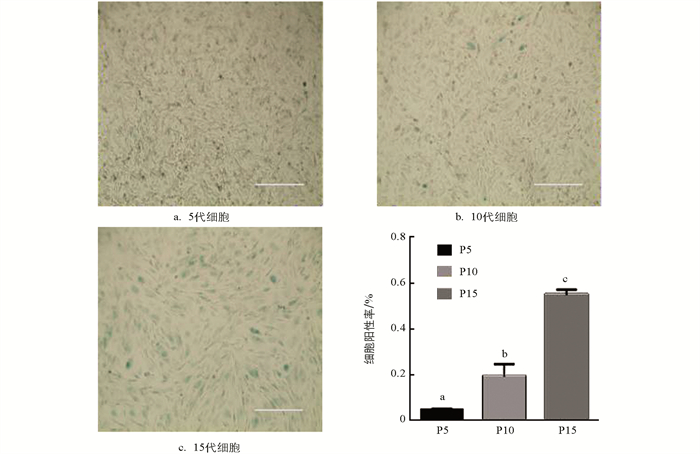
对培养的5,10,15代水牛成纤维细胞进行β-半乳糖苷酶染色,结果显示随着细胞代数增加,β-Gal细胞阳性率升高,不同代数之间差异具有统计学意义(p < 0.05),其中15代细胞的衰老程度最严重(图 5). qRT-PCR结果显示,与衰老相关的P16基因表达随细胞代数增加逐渐升高,不同代数间差异具有统计学意义(p < 0.05);P21基因表达水平从5代开始逐渐升高,并且5,10,15代之间差异具有统计学意义(p < 0.05);与0代相比,5,10,15代P53基因的表达均显著降低(p < 0.05),但5,10,15代之间差异不具有统计学意义(p > 0.05)(图 6).
-
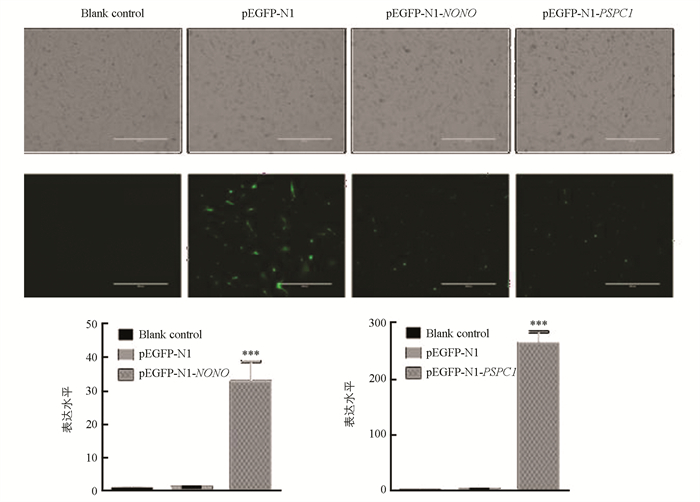
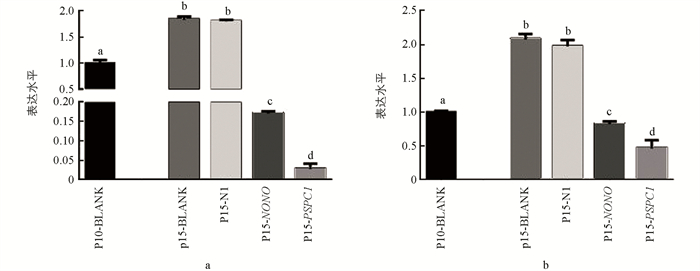
将pEGFP-N1-NONO和pEGFP-N1-PSPC1质粒分别转染第10代水牛胎儿成纤维细胞,48 h后在荧光显微镜下观察转染效果(图 7). 收集P15代细胞,利用qRT-PCR技术检测NONO和PSPC1表达水平(图 7). 结果显示,空白组细胞没有绿色荧光,pEGFP-N1空载组、pEGFP-N1-NONO和pEGFP-N1-PSPC1试验组都可以观察到绿色荧光. 与空白对照组和阴性对照组相比,NONO和PSPC1在试验组细胞中的表达差异具有统计学意义(p < 0.001).
-
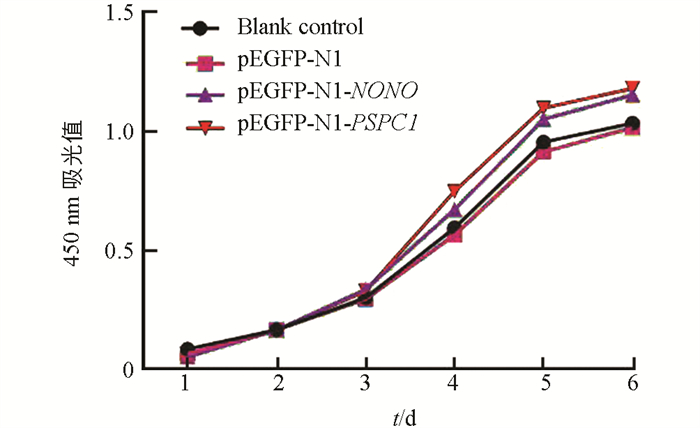
上述转染试验后,绘制15代水牛胎儿成纤维细胞生长曲线(图 8). 与空白对照组和阴性对照组相比,两个试验组细胞增殖速度均加快;与NONO试验组相比,PSPC1试验组细胞的增殖速度更快. qRT-PCR结果显示,凋亡相关基因在空白对照组与阴性对照组中的表达差异不具有统计学意义(p > 0.05). 与对照组相比,Bax在两个试验组的表达显著降低(p < 0.05);Bcl-2在两个试验组的表达差异不具有统计学意义(p > 0.05);Bax在PSPC1试验组细胞中的表达显著低于NONO试验组(p < 0.05)(图 9).
-
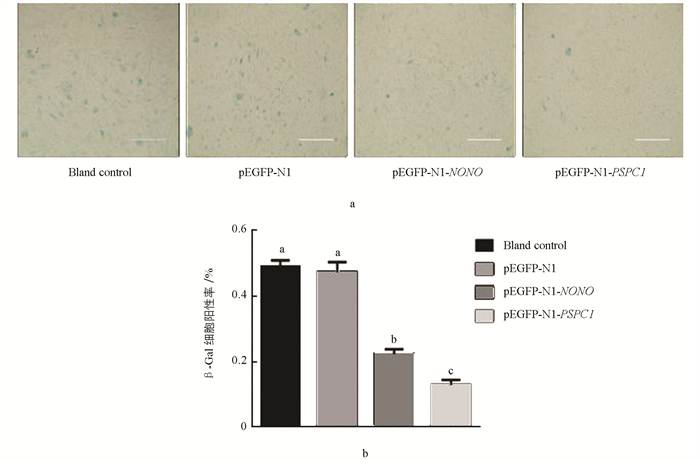
对转染的15代水牛胎儿成纤维细胞进行β-半乳糖苷酶染色. 结果显示,空白对照组与阴性对照组差异不具有统计学意义(p > 0.05);与对照组相比,2个试验组β-Gal细胞阳性率显著降低,其中PSPC1试验组显著低于NONO试验组(p < 0.05)(图 10). qRT-PCR结果显示,P16在空白对照组与阴性对照组细胞中的表达差异不具有统计学意义(p > 0.05);与对照组相比,两个试验组细胞中P16表达显著降低,其在PSPC1试验组的表达显著低于NONO试验组(p < 0.05)(图 11).
2.1. 水牛NONO和PSPC1基因编码区的克隆及序列分析
2.2. 旁粒相关基因在不同代数水牛胎儿成纤维细胞中的表达
2.3. 不同代数水牛胎儿成纤维细胞衰老及相关基因的表达分析
2.4. 过表达NONO和PSPC1对水牛胎儿成纤维细胞增殖及衰老的影响
2.4.1. 过表达NONO和PSPC1效果检测
2.4.2. 过表达NONO和PSPC1对水牛胎儿成纤维细胞增殖的影响
2.4.3. 过表达NONO,PSPC1对水牛胎儿成纤维细胞衰老及相关基因表达的影响
-
以往的研究表明,旁粒相关基因NONO和PSPC1在DNA损伤修复、细胞凋亡、细胞增殖等方面均发挥相关作用,其在细胞增殖方面的作用与细胞种类有关. 本研究首次从旁粒角度研究其在体外培养的水牛成纤维细胞的增殖及抗衰老作用,发现旁粒相关基因NONO和PSPC1可以提高水牛体细胞增殖能力,延缓衰老. 本研究结果为调节水牛供体细胞生理状态,进一步提高水牛体细胞克隆效率奠定了基础.
一般体细胞克隆供体细胞的代数会控制在10代以内,最好不超过15代[17]. 本研究在细胞衰老模型构建时,选择5代、10代和15代水牛胎儿成纤维细胞进行筛选. 结果发现,随着细胞代数增长,细胞生长活力逐渐减弱,β-Gal细胞阳性率显著上升,衰老细胞占总细胞数增加,细胞整体趋向于衰老. 结合文献和试验结果,选择10代作为细胞衰老的处理点用于后续试验[18-20]. P16,P21,P53是常见的用于检测细胞衰老指标[21-25],P16在人和小鼠中属于较为可信的体内衰老标记物[26],而P21基因和P53基因表达的增加不能具体区分诱导原因是衰老还是凋亡,所以必须对水牛胎儿成纤维细胞衰老标记物的选择进行筛选. 选择0代、5代、10代和15代水牛胎儿成纤维细胞进行基因表达分析,结果显示随着细胞代数增加,P16基因和P21基因的表达呈现出上升趋势;P53在0代的表达显著高于其他代数,5代到15代差异不具有统计学意义. 因此,选择P16和P21作为水牛胎儿成纤维细胞衰老标记物进行分析.
选择10代水牛胎儿成纤维细胞,利用基因过表达技术及后续检测来验证本文的推论. 对15代细胞进行β-半乳糖苷酶染色,发现2个试验组β-Gal细胞阳性率显著降低,其中PSPC1试验组显著低于NONO试验组. Gao等[10]发现敲低PSPC1会导致更多细胞进入G2/M期,而过表达PSPC1则会使细胞聚集在G1/S期,本文在绘制15代细胞生长曲线时,也发现在第3~5 d时PSPC1试验组的细胞增殖速度高于其他组. 分析15代细胞的凋亡相关基因,结果显示与对照组相比,试验组的Bax表达显著降低,其中PSPC1试验组Bax表达显著低于NONO试验组. 本文推测,NONO和PSPC1表达与细胞增殖呈正相关,这与Cheng等[11]在食管鳞状细胞癌细胞、Alfano等[12]和Gao等[10]在HeLa细胞上的研究结果一致. P16和P21表达检测结果显示,两个实验组β-Gal细胞阳性率都显著降低,其中PSPC1实验组显著低于NONO实验组;两个实验组中P16,P21的表达水平也都降低,并且PSPC1实验组的表达水平显著低于NONO实验组,可见过表达NONO和PSPC1基因能够缓解细胞衰老.
-
本研究克隆得到了水牛NONO和PSPC1基因编码序列. 上调NONO和PSPC1基因表达能够促进水牛胎儿成纤维细胞增殖并且延缓细胞衰老,其中PSPC1比NONO基因的作用更加明显.








 DownLoad:
DownLoad: