-
开放科学(资源服务)标识码(OSID):

-
番茄(Solanum lycopersicum)是仅次于土豆的世界第二大消费蔬菜[1],而由茄链格孢菌(Alternaria solani)侵染引起的番茄早疫病造成的经济损失高达70%~80%[2]. 因此,加强番茄早疫病的防治研究,对降低番茄早疫病、促进农业生产、提高农民收入具有重要意义.
生物防治具有绿色、安全的特性,利用拮抗微生物防治植物病害是生物防治的一个重要方面[3]. 海洋是最多样和最具竞争力的生态系统[4],如研究发现海洋发根链霉菌和解淀粉芽孢杆菌可以作为番木瓜中抵抗炭疽病的抗性诱导剂[5]. 孙一凡等[6]发现番茄植株经侧孢短芽孢杆菌(Brevibacillus laterosporus)B113处理后,叶片中多酚氧化酶(Polyphenol oxidase,PPO)、过氧化物酶(Peroxidase,POD)等防御酶活性提高,同时番茄早疫病对植株的侵染程度降低. 唐彤彤等[7]发现赤杆菌(Erythrobacter)YH-07对番茄枯萎病有很好的防治效果. 目前,针对番茄早疫病可供选择的菌株资源仍相对较少,仍需大量筛选及鉴定对番茄病害广谱、高效拮抗的菌株.
本实验室前期从连云港海域条斑紫菜附生菌中筛选到一株对番茄早疫病菌有强烈抑制作用的海洋细菌221-11. 本研究拟对该菌株进行种属鉴定,并通过盆栽试验探究此拮抗菌对番茄的促生作用和对番茄早疫病的防治效果,为海洋细菌在生物防治中的应用打下理论基础.
HTML
-
番茄早疫病菌系茄链格孢菌来自中国农业科学院生物防治研究所,本实验室保存. 拮抗菌为本实验室从连云港海域中的条斑紫菜表面分离,编号221-11. 供试番茄来源于宿迁市沭阳大棚蔬菜种植基地购买的长势及各项生长指标相近、对早疫病原菌无抗性的健康番茄苗.
-
革兰氏染色、芽孢染色和鞭毛染色试剂盒购自青岛高科技工业园海博生物技术有限公司,酶活试剂盒购自南京建成生物工程研究所,其他生化试剂为国产分析纯.
-
采用平板对峙法测定海洋拮抗菌221-11对茄链格孢菌的抑菌作用,对照组与处理组各重复3次,于28 ℃生化培养箱培养7 d后测量并计算抑制率. 菌丝生长抑制率(%)=(对照组菌落直径-处理组菌落直径)/(对照组菌落直径-菌饼直径)×100%[11].
-
1) 形态学观察. 采用平板划线法将拮抗菌接种于PDA培养基,于28 ℃恒温培养24 h,观察并记录平板上菌株的形态. 同时采用革兰氏染色、孢子染色和鞭毛染色方法进行进一步观察.
2) 生理生化特征观察. 参照《微生物学实验》[12]和《伯杰细菌鉴定手册》[13],将菌株221-11分别接种于纤维素刚果红培养基等培养基上,分别观察是否有透明圈产生. 鉴定菌株221-11对ONPG、葡萄糖等营养物质的利用能力.
3) 分子生物学鉴定. 利用16S rDNA序列通用引物27F:5′-AGAGTTTGATCMTGGCTCAG-3′和1492R:5′-GGTTACCTTGTTACGACTT-3′参照已报道的方法[14]进行基因扩增并在上海生工生物工程有限公司进行测序,将所测得的16S rDNA序列与GenNBank中已知的序列进行同源性分析,选择相似度最高的同源序列,用MEGA-11.0软件进行Clustal W多重序列比对,并采用NJ(Neighbour-Joining)算法构建系统发育树.
-
将拮抗菌221-11置于28 ℃下,在PDA培养基划线培养48 h后接种到PDB培养基中,恒温振荡培养(28 ℃,转速180 r/min)24 h后,用无菌水100倍梯度稀释配成5.75×102~5.75×108 CFU/mL的系列发酵液. 取4 ℃冰箱保存的番茄早疫病菌于PDA培养基上,28 ℃培养5 d后制成直径6 mm的菌饼,备用.
-
将1.2.3中稀释的发酵液浇灌到幼苗根部,同时设置清水为对照组,每处理组10株,每株10 mL,每7 d灌根一次,共5次. 培养35 d后将植株连根拔起,洗净根部的土壤和肥料,吸水纸吸干水分,分别统计株高、鲜质量和干质量[15].
-
拮抗菌221-11对番茄早疫病的防效测定采用盆栽试验. 取纯化后的番茄早疫病菌,用打孔器打下制成菌饼. 在预防测定中,将10 mL浓度为5.75×108 CFU/mL的发酵液均匀喷洒在植株叶片上,保湿1 d后再将番茄早疫病菌菌饼倒扣在番茄叶片中央;治疗测定中,先接种病菌,保湿1 d后再均匀喷洒5.75×108 CFU/mL发酵液. 以清水为对照组,每组处理设置20株幼苗,重复3次[16-17]. 在25 ℃、12 h∶12 h光周期条件下保湿培养7 d后,测定3组的发病率和病情指数.
病情分级标准与计算方式是以叶片为单位,0~4级为标准调查番茄幼苗的病情指数[18]. 病情指数=∑(各级病叶数×对应发病级数)/(调查总叶片数×最高调查发病级数)×100;预防效果(治疗效果)=(对照病情指数-处理病情指数)/对照病情指数×100.
-
分别以灌根的方式浇灌10 mL 5.75×104 CFU/mL、5.75×108 CFU/mL浓度的海洋拮抗菌221-11发酵液到幼苗根部,以无菌水为对照. 每5 d灌根一次,10 d后采集植株叶片(倒数第1和倒数第2的完全展开叶)进行研磨,得到粗酶液. 按照蔡庆生[19]的方法测定SOD、POD和CAT活性.
-
试验数据采用SPSS 27.0进行数据分析,以最小显著差数法(LSD)分析差异显著性.
1.1. 材料
1.1.1. 供试菌株和植物来源
1.1.2. 供试培养基
1.1.3. 供试试剂
1.2. 试验方法
1.2.1. 海洋拮抗菌221-11的抑菌作用
1.2.2. 海洋拮抗菌221-11的鉴定
1.2.3. 海洋拮抗菌221-11发酵液和番茄早疫病原菌的制备
1.2.4. 海洋拮抗菌221-11对番茄幼苗的促生作用
1.2.5. 海洋拮抗菌221-11对番茄早疫病的防效测定
1.2.6. 海洋拮抗菌221-11对番茄幼苗叶片防御酶活性的影响
1.3. 数据统计与分析
-
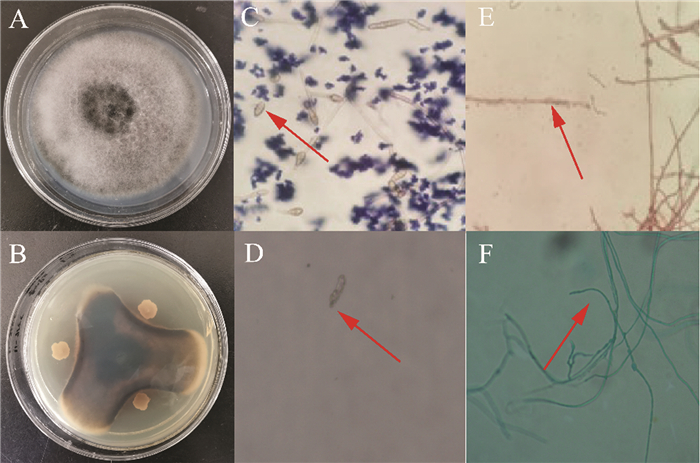
海洋细菌221-11对番茄早疫病菌具有强烈抑菌作用(图 1A和B). 抑菌带直径为61.2±0.3 mm,菌丝生长抑制率为77.2%. 与正常生长的孢子和菌丝相比(图 1C和E),受抑制的孢子呈现长棒状、细长且出现破裂,菌丝则变得膨大、弯曲、分枝增多(图 1D~F).
-
海洋拮抗菌221-11菌体为杆状,革兰氏染色为阳性;在PDA培养基上单菌落为乳白色、不透明、边缘不整齐、表面干燥和褶皱,芽孢呈绿色,鞭毛呈紫色(图 2),属于芽孢杆菌属.
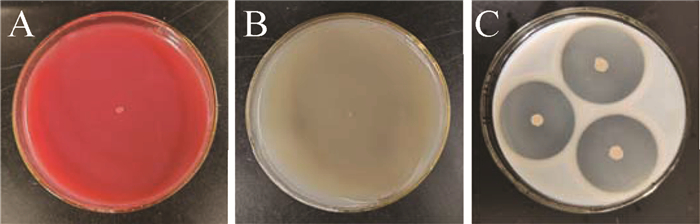
海洋拮抗菌221-11生理生化特征测定结果见表 1和图 3,该菌能够利用部分糖类,产蛋白酶,透明圈直径为37.2±0.2 mm(图 3).
-
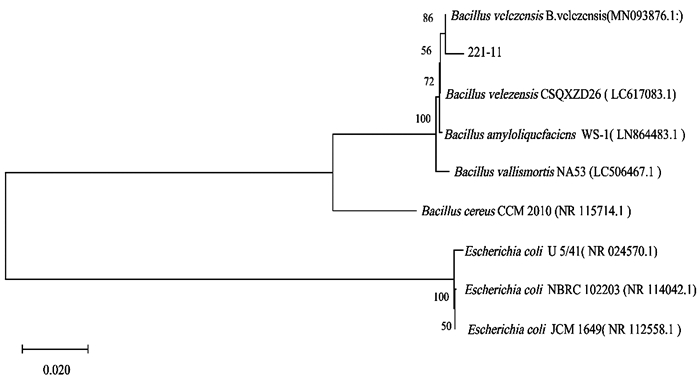
16S rDNA测序所得序列经网上比对发现与贝莱斯芽孢杆菌(GenBank号:MN093876)相似度最高,相似度为99%. 系统发育树发现海洋拮抗菌221-11序列与贝莱斯芽孢杆菌(Bacillus velezensis)聚为一支(图 4). 再结合形态学观察和生理生化鉴定结果,确定拮抗菌221-11为贝莱斯芽孢杆菌(B. velezensis).
-
灌根35 d后,株高与质量如表 2所示. 结果发现不同浓度海洋拮抗菌221-11菌液对番茄植株的生长均有一定的促进作用,各生长指标与对照相比均显著差异(p < 0.05). 当发酵液浓度为5.75×108 CFU/mL时,番茄的株高、鲜质量和干质量达到最大值,增长率分别为15.23%、64.23%和118.97%;同时鲜质量和干质量与其他处理组及对照组间差异均达到极显著水平(p < 0.001).
-
研究结果表明221-11可显著降低病情指数,治疗组和预防组的病情指数分别比对照组降低45.73%和63.36%,并且预防效果要高于治疗效果(表 3).
-
不同浓度的拮抗菌221-11对番茄植株内相关防御酶活性均有一定的增加. SOD、POD和CAT活性都随着菌液浓度(0、5.75×104和5.75×108 CFU/mL)的增大而升高,当发酵液浓度为5.75×108 CFU/mL时达到最高,酶活性分别为对照的119.24%、158.82%和239.82%,POD、CAT活性与对照组相比差异极显著(p < 0.001)(表 4).
2.1. 海洋拮抗菌221-11的抑菌作用
2.2. 海洋拮抗菌的鉴定
2.2.1. 形态学观察和生理生化特征
2.2.2. 海洋拮抗菌221-11的16S rDNA序列分析
2.3. 海洋拮抗菌221-11对番茄幼苗的促生作用
2.4. 海洋拮抗菌221-11对番茄早疫病的防治效果
2.5. 海洋拮抗菌221-11对番茄叶片防御酶活性的影响
-
有研究表明,从海洋环境中分离得到的细菌,能够产生多种抗微生物的活性物质,并诱导宿主产生抗性[20-22],在植物抵御植物病原体方面发挥着越来越重要的作用,发掘海洋微生物资源充实抗菌微生物库成为生物防治的主要任务之一. 本试验从海洋条斑紫菜表面分离的贝莱斯芽孢杆菌221-11经过平板对峙培养,结果表明对番茄早疫病菌等植物病菌具有显著抑制作用,丰富了番茄早疫病微生物生物防治的菌种资源.
相对于放线菌和真菌来说,芽孢杆菌生成的芽孢具有较强的抗逆性、容易生产、需要营养简单、易于保存和使用等优点,所以市场上商业化的拮抗制剂大多为芽孢杆菌属[23]. 海洋芽孢杆菌属细菌,如解淀粉芽孢杆菌(Bacillus amyloliquefaciens)[24]、枯草芽孢杆菌(Bacillus subtilis)[25]、贝莱斯芽孢杆菌(B.velezensis)[26]、苏云金芽孢杆菌(Bacillus thuringiensis)[27]等,已经被证实是一种植物生长促进剂和免疫系统性诱导剂,能产生多种抗微生物化合物,抑制病原菌生长. 沈会芳等[28]的试验表明,贝莱斯芽孢杆菌GDND能抑制烟草镰孢根腐病菌的整个生产发育过程,包括孢子萌发、菌丝生长和孢子形成. 本研究从连云港海域条斑紫菜表面筛选到的番茄早疫病菌拮抗菌221-11初步鉴定为贝莱斯芽孢杆菌,能强烈地抑制番茄早疫病菌的生长,具有进一步的应用价值.
白红燕等[29]通过温室试验和大田试验发现贝莱斯芽孢杆菌EBV02对棉花黄萎病的防治效果分别可达68.33%和37.25%,且可以显著促进棉苗生长;安婉宁等[30]从渤海海泥分离筛选出的贝莱斯芽孢杆菌CT2628可以增强植物的保水能力和光合作用,促进植物生长. 本研究与前人研究结果一致,盆栽试验表明贝莱斯芽孢杆菌221-11具有显著的促生长和防治效果. 贝莱斯芽孢杆菌221-11在试验浓度下均能够促进番茄植株生长,鲜质量和干质量与对照相比差异极显著.
SOD、POD与CAT可调节H2O2浓度,降低植物细胞膜脂的过氧化作用,保护植物细胞,避免病原体进一步侵害植物[31-32]. 邹强等[33]指出接种TP-1发酵液可以诱导葡萄相关防御酶活性的提高,增强葡萄对灰霉病的抗性. 赵欣等[34]发现玉米幼苗接种解淀粉芽孢杆菌HRH317后,其叶片内SOD、POD和CAT活性峰值是对照的1.38~3.35倍. 本项研究中灌根海洋拮抗菌221-11发酵液后,叶片防御酶SOD、POD、CAT的活性随着发酵液浓度的升高而增强,CAT和POD活性表现为极显著差异,SOD表现为显著差异,与赵欣等研究结果[34]相似. 然而本试验CAT酶活(2.40倍)增加最多,SOD增加的效果最差;赵欣等[34]的研究中SOD增加效果最高(3.35倍),POD增加最少(1.38倍),这可能与拮抗菌的种类与特性有关.
本研究结果也存在一些不足. 据前人研究,拮抗菌可以在土壤及植物组织内进行定殖从而增强植物生理活动,诱导非特异性防御酶增加. 本研究在促生与防御酶酶活测定试验中采用灌根方式,而作为药剂,在实际操作中喷洒操作更为常见,因此在防效试验中应采用喷洒方式. 关于221-11的最佳使用方式还需要进一步研究,同时要明确防治因子的具体种类和作用机制[35-36]、拮抗菌在土壤中的定殖效果[37-38]、定殖的影响因素以及菌剂制备方法和使用方法,并进一步做更加深入的研究.






 DownLoad:
DownLoad: