-
果胶是由多缩半乳糖醛酸甲酯通过β-1,4糖苷键与钙、镁结合成的天然高分子化合物,主要存在于果皮中,在食品工业中应用广泛,也是医药和化妆品重要的生产辅料[1-5].硝酸盐(NO3-)和亚硝酸盐(NO2-)是引起人们食物中毒的常见物质,亚硝基化合物中的NO2-能与食品中存在的仲胺、叔胺发生一系列反应,形成能够导致癌变的亚硝胺[6].据研究,膳食纤维对体内的NO2-有一定的吸附能力,能防止胃癌、心脏病等一些疾病的发生[7-11],其中,麦麸、米糠膳食纤维对NO2-的体外吸附效果明显.而果胶作为水溶性膳食纤维,近年来已有学者利用果胶吸附去除水体中Pb2+,Cu2+,Hg2+等离子[12-16],但对果胶吸附NO2-的研究尚无报道.本实验基于果胶的凝胶、吸附等特性,拟在模拟胃环境下研究柚子白皮果胶对NO2-的吸附动力学特性,确定果胶对NO2-的吸附性能,为开发NO2-体内清除剂提供新思路.
全文HTML
-
柚子皮,由漳州市平和县蜜柚种植基地提供;市售果胶,上海阿拉丁生物科技股份有限公司.
-
HQY-C型恒温振荡摇床,金坛市鸿科仪器厂;UV-1100紫外分光光度计,上海美谱达仪器有限公司;AR124W电子天平,奥豪斯仪器(上海)有限公司;80-2电动离心机,金坛市科析仪器有限公司;NICOLET iS 10型傅里叶红外分光光度计,美国赛默飞世尔公司;EL20型pH计,梅特勒-托利多仪器上海有限公司;DUG-9030A干燥箱,上海精宏实验设备有限公司;JHBE-50S闪式提取器,西安太康生物科技有限公司;RE-301旋转蒸发器,巩义市予华仪器有限责任公司;RE-301恒温水油浴锅,巩义市予华仪器有限责任公司;RE-52AA旋转蒸发器,上海亚荣生化仪器厂;SHB-Ⅲ循环水式多用真空泵,郑州长城科工贸有限公司;HH-2数显恒温水浴锅,金坛市科析仪器有限公司;EG323LC8-NS微波炉,广东美的电器股份有限公司;手持式折光仪,上海光学仪器厂;FW-100高速万能粉碎机,天津市泰斯特仪器有限公司.
-
新鲜柚皮,去除黄色表皮,将柚子白皮剪成3~4 mm大小,按液料比15:1(mL/g)加入去离子水,设置闪式提取器电压100 V,闪式破碎60 s,用HCl(1+1)调节酸解液pH值至1.5,微波加热至微沸,置于80 ℃下回流提取30 min后,趁热用300目滤布抽滤,将滤液以75 ℃,70 r/min旋转蒸发浓缩至折光率为5%,冷却,用稀氨水调节浓缩液pH值至4.5,在不断搅拌下加入2倍体积的95%乙醇,放置12 h使之沉淀,用300目滤布双层抽滤,除去乙醇后,再用乙醇洗涤2次,抽滤,加入2倍体积蒸馏水加热溶解,浓缩至折光率为5%,冷却,-18 ℃预冻,70 Pa条件下真空冷冻干燥(加热板温度40 ℃),得果胶(半乳糖醛酸质量分数78.7%,酯化度77.4%),粉碎后备用[17].
-
取3.2 g胃蛋白酶和2.0 g的固体NaCl溶于1 000 mL去离子水中,用浓HCl调节至一定pH值,制成模拟胃液[18].
-
柚皮果胶添加量为0.4 g/L,设置模拟胃液中NO2-质量浓度分别为5 mg/L,10 mg/L,15 mg/L,20 mg/L,25 mg/L,30 mg/L,pH值调节至2,设置吸附时间分别为10 min,20 min,40 min,60 min,80 min,100 min,120 min,150 min,180 min,210 min,240 min,300 min,360 min,420 min,480 min,测定柚子白皮果胶对NO2-的吸附量和去除率.
-
柚皮果胶添加量为0.4 g/L,模拟胃液NO2-质量浓度为30 mg/L,调节pH值分别为1,1.5,2,2.5,3,吸附时间420 min,测定柚子白皮果胶对NO2-的吸附量和去除率.
-
设置柚皮果胶添加量分别为0.2 g/L,0.4 g/L,0.6 g/L,0.8 g/L,1.0 g/L,模拟胃液NO2-质量浓度为30 mg/L,pH值调节至1.5,吸附时间420 min,测定柚皮果胶对NO2-的吸附量和去除率.
-
柚子白皮果胶和市售果胶添加量为0.8 g/L,NO2-质量浓度为30 mg/L,pH值调节至1.5,吸附时间420 min,测定柚皮果胶和市售果胶对NO2-的吸附量和去除率.
-
取柚子白皮果胶,按一定液料比加入含一定质量浓度NO2-的不同pH模拟胃液中,于37 ℃环境中模拟人体胃的蠕动,以100 r/min恒温振荡使其吸附一定时间后,于4 000 r/ min离心10 min,上清液采用盐酸萘乙二胺法[19-20]测NO2-残留量,计算吸附量(式1)和去除率(式2),计算公式[21]如下:
吸附量:
去除率:
式中,q为吸附量,mg/g;∂为去除率,%;C0为吸附前的NO2-质量浓度,mg/L;Ce为吸附后的NO2-质量浓度,mg/L;V为NO2-溶液体积,L;m为果胶添加量,g.
-
将柚子白皮果胶和市售果胶按照最佳条件进行吸附后,加入2倍体积无水乙醇沉淀12 h,抽滤,干燥后,进行红外光谱分析,测试条件:波数范围4 000~500 cm-1中红外,扫描次数为15,分辨率4 cm-1,DTGS检测器.
1.1. 实验材料
1.2. 仪器和设备
1.3. 实验方法
1.3.1. 柚子白皮果胶的制备
1.3.2. 模拟胃液的制备
1.3.3. 实验设计
1.3.3.1. NO2-初始质量浓度、吸附时间对吸附效果的影响
1.3.3.2. pH值对果胶吸附NO2-效果的影响
1.3.3.3. 柚皮果胶添加量对NO2-吸附效果的影响
1.3.3.4. 柚皮果胶和市售果胶对NO2-吸附效果的影响
1.3.4. 测定方法
1.3.5. 果胶吸附前后的红外测定
-
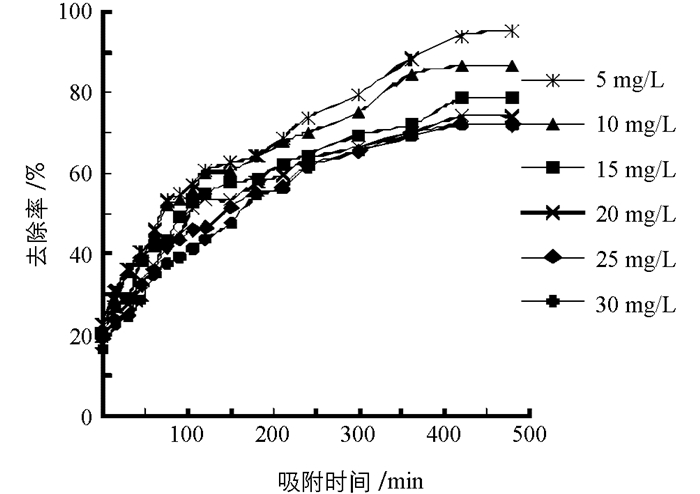
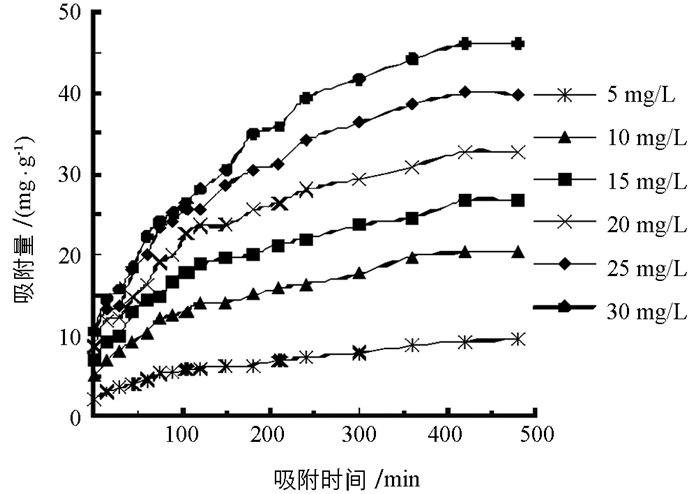
柚子白皮果胶对NO2-的吸附量和去除率如图 1、图 2.由图可知,模拟胃环境下,NO2-初始质量浓度一定时,柚子白皮果胶对NO2-的吸附量和去除率均随着吸附时间的延长而显著增加,吸附420 min后,果胶对NO2-的吸附量和去除率趋于平缓并达到吸附平衡;同时,随着NO2-初始质量浓度的增大,柚子白皮果胶对NO2-的吸附量逐渐增大,去除率逐渐降低,当NO2-初始质量浓度为5 mg/L时,平衡吸附量为9.3 mg/g,去除率为94.1%,而当NO2-初始质量浓度为30 mg/L时,平衡吸附量为46.1 mg/g,去除率仅为72.1%.由此可知,NO2-初始质量浓度越大,柚子白皮果胶对NO2-的平衡吸附量越大,去除率越低. WTO/FAO规定人体NO2-的日允许摄入量为4 mg/kg[22],经过换算接近于30 mg/L,因而选择此浓度为最佳初始质量浓度.
-
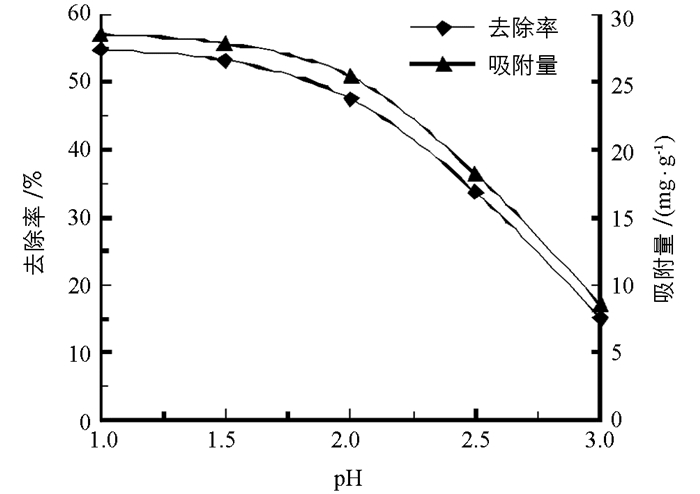
由图 3可知,吸附环境酸性越强,柚子白皮果胶对NO2-的吸附量越大,去除率越高.当pH值从1增大到1.5时,柚皮果胶对NO2-的吸附量和去除率缓慢下降,当模拟胃环境pH值大于1.5,随着pH值的逐渐增大,柚皮果胶对NO2-的吸附量和去除率急速下降. pH值为1时,柚皮果胶对NO2-的吸附量为28.6 mg/g,去除率为54.9%;pH值为1.5时,吸附量为27.9 mg/g,去除率为53.2%;pH值为3时,吸附量仅为8.55 mg/g,去除率仅为15.0%.由此可知,反应体系的pH值对柚皮果胶吸附NO2-的影响较大,pH值越低吸附效果越好,pH值为1.5时柚子白皮果胶模拟胃环境下对NO2-的吸附量和去除率较高,也较接近真实胃环境pH值.
-
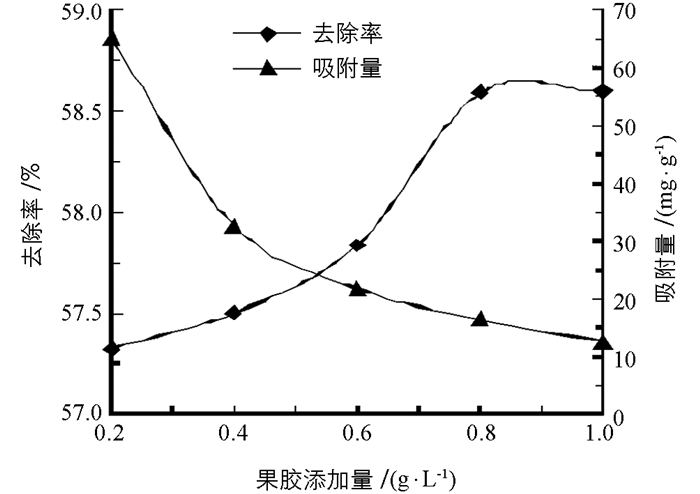
柚皮果胶对NO2-的吸附量迅速降低,去除率急剧升高,当柚皮果胶添加量大于0.8 g/L,去除率达到最大并趋于稳定(图 4). 0.2 g/L柚皮果胶对NO2-的吸附量为65.1 mg/g,去除率为57.3%;而0.8 g/L柚皮果胶对NO2-吸附量为16.4 mg/g,去除率为58.6%;果胶的添加量增加到1 g/L时,吸附量仅为12.5 mg/g,去除率同为58.6%,说明增加柚皮果胶添加量,吸附活性位点增加,NO2-去除率增大,但单位体积柚皮果胶对NO2-的吸附量降低.果胶添加量为0.8 g/L时,柚子白皮果胶对NO2-的去除率最高.
-
由表 1可知,柚皮果胶与市售果胶对NO2-吸附量和去除率相当,由此可知,从柚子白皮提取的果胶对NO2-的吸附效果与市售果胶近似相等,产品质量可靠.
-
柚皮果胶对NO2-的吸附动力学可用Lagergren准一级动力学模型、准二级动力学模型、颗粒内扩散模型、Elovich模型和Bangham模型进行拟合,并通过线性拟合参数进行描述.各模型方程如下[23-24]:
准一级动力学模型:
准二级动力学模型:
颗粒内扩散模型:
Elovich模型:
当abt≫1时,令
则得到Elovich方程的线性形式为
Bangham模型:
式中,qe为平衡吸附量,mg/g;qt为t时刻的吸附量,mg/g;K1为准一级吸附速率常数[23];K2为准二级吸附速率常数;Kt为颗粒内扩散速率常数;C为颗粒内扩散方程常数[24];a为常数;b为速率常数;K0为吸附速率常数;α为常数.
-
分别利用上述5种动力学模型对果胶吸附NO2-的实验数据进行拟合,再对拟合所得曲线进行线性回归分析,求出各个模型的动力学参数及线性相关系数R2(表 2).
由表 2可知,果胶吸附NO2-的颗粒内扩散模型R2在0.969 8~0.990 0之间,其线性相关系数较高,说明颗粒内扩散模型能够较好地拟合柚子白皮果胶对NO2-的吸附过程,吸附前期NO2-向果胶外表面扩散,吸附中后期为NO2-的内扩散,果胶孔隙中的溶液扩散和孔隙内表面的二维扩散过程随吸附反应的进行逐渐达到吸附平衡[21, 25-26];NO2-初始质量浓度越大,C值越大,果胶边界层厚度对NO2-吸附效果的影响也越大;Kt与C越大,说明果胶吸附NO2-的速率越快,吸附越容易达到平衡.
-
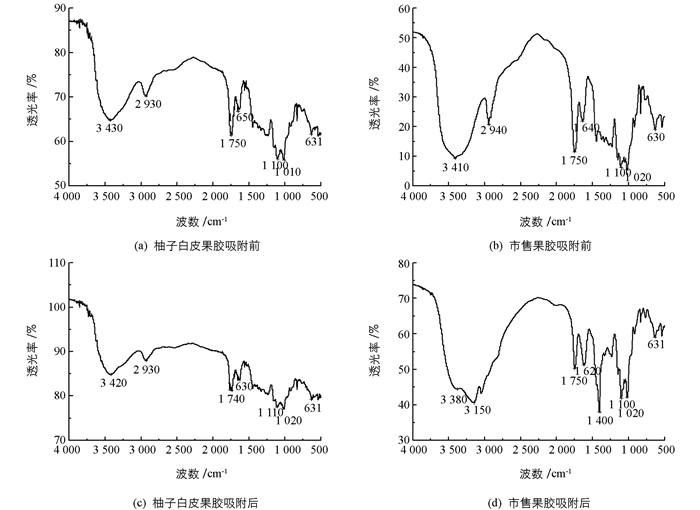
图 5反映了柚子白皮果胶与市售果胶在吸附NO2-前后的红外光谱变化.由图 5可以看出,柚子白皮果胶与市售果胶的功能基团基本一致.其中在3 430 cm-1附近较宽的吸收峰为O—H以及—NH2的伸缩振动峰,2 940 cm-1附近为—CH2的伸缩振动,1 750 cm-1为典型的羰基C=O伸缩振动峰,这可能是果胶中的羧酸或酯引起的,1 650 cm-1处吸收峰位于“酰胺Ⅰ带”,是果胶中乙酰氨基的C=O对称伸缩振动引起的;1 100~1 000 cm-1间的吸收峰是由C—O—H和糖环C—O—C的C—O伸缩振动引起的.吸附NO2-后,柚子白皮果胶与市售果胶在3 430 cm-1处吸收峰均发生了明显位移,这可能是由于O—H与NO2-结合后,吸收峰向低波长方向移动;另外,从图中可以观察到,吸附NO2-后,柚子白皮果胶在1 750 cm-1处的羰基吸收峰发生位移,表明果胶中的羧酸和/或酯也参与了对NO2-的吸附;而市售果胶乙酰氨基的C=O伸缩振动峰的相对强度发生显著变化,这意味着羧酸和/或酯与NO2-结合后发生了氨基化现象,同时也表明柚子白皮果胶与市售果胶的分子组成基本一致,但具体的成分仍然略有区别[27-29].
2.1. NO2-初始质量浓度、吸附时间对吸附效果的影响
2.2. pH值对果胶吸附NO2-效果的影响
2.3. 柚皮果胶添加量对NO2-吸附效果的影响
2.4. 柚皮果胶和市售果胶对NO2-吸附效果的影响
2.5. 柚子白皮果胶对NO2-的吸附动力学
2.5.1. 吸附动力学方程
2.5.2. 柚子白皮果胶对NO2-的吸附动力学分析
2.6. NO2-吸附前后柚子白皮果胶与市售果胶的红外光谱分析
-
1) 柚子白皮果胶对NO2-的吸附性能受果胶添加量、NO2-浓度、模拟胃环境pH值、吸附时间等因素的影响;柚皮果胶添加量与NO2-的单位吸附量呈负相关,而吸附时间、NO2-的浓度、模拟胃环境的pH值与NO2-的单位吸附量则呈正相关;当果胶添加量为0.8 g/L,pH值为1.5,NO2-质量浓度为30 mg/L,吸附时间为420 min时,果胶对NO2-的去除率达到56.21%,吸附量达17.65 mg/g.
2) 采用准一级动力学模型、准二级动力学模型、Evolich模型、Bangham模型、颗粒内扩散模型分别对柚皮果胶吸附NO2-的数据进行拟合,发现颗粒内扩散模型能够较好地拟合柚子白皮果胶对NO2-的吸附过程,NO2-初始质量浓度越大,C值越大,果胶边界层厚度对NO2-吸附效果的影响也越大;Kt与C的值越大,说明果胶吸附NO2-的速率越快,吸附越容易达到平衡.
3) 柚子白皮果胶和市售果胶吸附NO2-前后在3 400 cm-1,1 750 cm-1,1 650 cm-1处的吸收峰发生了明显的偏移和强度的变化,说明吸附前后柚皮果胶和市售果胶的功能基团基本相似,羟基、羰基(羧酸和/或酯)等基团参与了果胶吸附NO2-的反应.



 下载:
下载: