-
牛蒡子是菊科植物牛蒡(Arctium lappa L.)的干燥成熟果实,又名恶实、大力子等,具有疏散风热、透疹解毒的功效,为我国传统中药[1].现代药理研究表明,牛蒡子具有抗肿瘤、降血糖、抗菌、抗病毒等活性[2].牛蒡子化学成分主要有木脂素、挥发油、油脂等,其中木脂素类成分能抑制血小板活化因子对血小板的结合作用[3-5].王宁、胥秀英等分别对牛蒡子中木脂素类和咖啡酰奎宁酸类化合物分离纯化与质量分数测定进行了系统研究[6-13].在此基础上,本实验以牛蒡子配方颗粒合格率为指标,采用正交优化实验进行了牛蒡子配方颗粒处方筛选及牛蒡子苷元的质量分数测定研究.
全文HTML
-
试剂:牛蒡子购于重庆市中药材市场;糊精、淀粉、乙醇为药用规格;牛蒡子苷元对照品购于成都普菲德生物技术有限公司(纯度≥98%);三氯甲烷,甲醇等为市售分析纯.
仪器:HPLC仪,岛津SPD20A.
1.1. 试药与仪器
-
取市售牛蒡子于60 ℃干燥3h,称取500 g 3份,分别加入8倍、5倍、3倍量的水,在80~90 ℃加热提取1 h,收集3次提取液,冷至室温,再上预先处理好的D101大孔吸附树脂柱,用75%乙醇洗脱,收集洗脱液浓缩干燥得牛蒡子提取物,备用.
-
精密称取不同比例的牛蒡子提取物粉末和辅料过100目筛,加入相应浓度的润湿剂(乙醇)制成软材,挤压过12目筛,湿颗粒在相应温度下恒温干燥30 min.得到干燥的牛蒡子颗粒.
-
以牛蒡子颗粒的合格率为指标,即可通过1号筛与不过5号筛的颗粒占颗粒总质量的百分比.
-
以原辅料比例、润湿剂的浓度、干燥温度为影响因素,每个因素3个水平,采用正交实验设计L9(34),筛选牛蒡子颗粒制剂的最优工艺.正交实验因素水平及实验结果见表 1与表 2.
取牛蒡子提取物按表 2加入辅料,混合,加入润湿剂,搅拌,制软材,制粒,干燥.计算颗粒合格率,筛选最佳制粒工艺,结果见表 2.
结果表明:根据综合评分结果,由直观分析可以得出影响牛蒡子颗粒的因素大小依次为B>C>A,结合直观分析,A3>A2>A1,选择A3;B3>B2>B1,选择B3;C3>C2>C1,选择C3;所以,最佳工艺参数为A3B3C3.即牛蒡子提取物、糊精、淀粉比例为3:6:1,加入润湿剂乙醇的浓度为85%,恒温干燥的温度为65 ℃.并且润湿剂的浓度对牛蒡子颗粒成型影响显著,牛蒡子与辅料的物料比对牛蒡子颗粒制剂成型的影响相对较小.结合方差分析(表 3)可知,B因素即乙醇浓度对结果影响有统计学意义,而物料比与干燥温度对结果影响无统计学意义.
-
按牛蒡子提取物、糊精、淀粉比例为3:6:1,加入润湿剂乙醇的浓度为85%,恒温干燥的温度为65 ℃,按“2.1”的制备方法制备3批供试品,测定颗粒合格率,结果表明该工艺稳定(表 4).
-
色谱柱:Dionex C18(250 mm×4.6 mm,5 μm);流动相:甲醇-水(40:60);流速:1.0 mL/min;检测波长:280 nm;柱温:室温.
-
精密称定牛蒡苷元对照品一定量,加入甲醇溶液溶解制成浓度为0.705 mg/mL的溶液,即得.
-
取牛蒡子配方颗粒适量,研磨成细粉,精密称取0.2 g,置具塞锥形瓶中,精密加入甲醇50 mL,密塞,称定质量,超声处理20 min,冷至室温,用甲醇补足减失质量,混匀,滤过,取续滤液,即得.
-
取缺牛蒡子的阴性样品0.2g,按“2.6.3”项下的方法制备,即得阴性对照溶液.
-
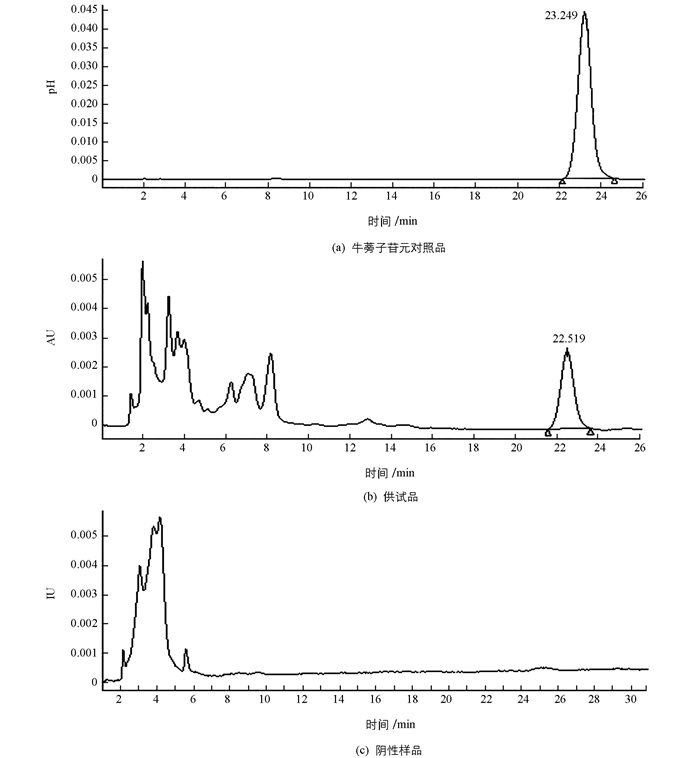
取“2.6.2”项下的对照品溶液,“2.6.3”项下的供试品溶液,“2.6.4”项下的阴性对照溶液各10 μL,按“2.6.1”项下的色谱条件测定,记录色谱图,结果见图 1.分离效果好,阴性样品无干扰.
-
精密吸取“2.6.2”项下对照品溶液2 μL,4 μL,6 μL,8 μL,10 μL,按“2.6.1”项下的色谱条件测定.以牛蒡子苷元的进样量(x,μg)为横坐标,以峰面积(y)为纵坐标,进行线性回归,得线性范围为1.41 μg~7.05 μg,回归方程为y=3×108x-139 413,相关系数r=0.999 9.
-
取“2.6.2”项下对照品溶液适量,按“2.6.1”项下色谱条件连续进样6次测定.结果牛蒡苷元峰面积的RSD为0.85%,表明仪器的精密度较好.
-
取同一供试品溶液,分别于0,2,4,6,8,12,24 h后进样测定.结果牛蒡苷元峰面积的RSD为1.06%,表明供试品溶液在24 h内稳定.
-
取同一样品6份,按“2.6.3”项下方法制备供试品溶液,再按“2.6.1”项下色谱条件测定.结果牛蒡苷元质量分数的RSD为0.74%,表明该方法的重复性比较好.
-
实验取牛蒡子样品3份,分别精密加入一定量牛蒡子苷元的对照品,按“2.6.3”项下的方法制备牛蒡子供试品溶液,再按“2.6.1”项下的色谱条件进样并且计算加样回收率为2.19%(表 5).
-
取3批次牛蒡子配方颗粒适量,分别按“2.6.3”项下方法制备供试品溶液,在“2.6.1”项下的色谱条件进样测定并计算其质量分数(表 6),结果表明通过该方法制备的样品牛蒡子苷元质量分数基本稳定.
2.1. 牛蒡子提取物的制备
2.2. 颗粒剂的制备
2.3. 评价指标
2.4. 正交实验设计
2.5. 正交设计验证实验
2.6. 牛蒡子配方颗粒中牛蒡子苷元质量分数测定研究
2.6.1. 色谱条件
2.6.2. 对照品溶液的制备
2.6.3. 供试品溶液的制备
2.6.4. 阴性对照溶液的制备
2.6.5. 专属性实验
2.6.6. 线性关系考察
2.6.7. 精密度实验
2.6.8. 稳定性实验
2.6.9. 重复性实验
2.6.10. 加样回收率
2.6.11. 样品质量分数测定
-
本实验是笔者团队在牛蒡子系统研究的基础上,采用水煮法结合大孔树脂精制牛蒡子提取物,并对牛蒡子配方颗粒的制备工艺进行了正交优化实验,筛选出牛蒡子配方颗粒的最优制备工艺:物料比即牛蒡子提取物、糊精、淀粉比例为3:6:1,乙醇的浓度为85%,干燥温度为65 ℃.并采用HPLC法对牛蒡子颗粒剂中牛蒡子苷元进行质量分数方法学研究及样品质量分数测定,结果表明,通过大孔树脂精制处理,可以大大提高牛蒡子配方颗粒中有效成分牛蒡子苷元的质量分数.该结果以期为牛蒡子的制剂开发提供思路.



 下载:
下载: