-
天然酶是一类生物催化剂,可高效、专一催化生化反应,而且反应条件温和.然而,天然酶易变性失活,提纯困难,价格昂贵,储藏和使用不便,成本高,所以模拟酶的研究应运而生.目前已有大量利用卟啉、主体试剂、印迹高分子、膜体系及配合物等作为模拟酶的报道[1].近年来,纳米材料模拟酶的研究引起广泛关注,各种各样的纳米材料,如金属氧化物、金属硫化物、金属纳米微粒及碳材料等均具有模拟酶特性[2].
金属有机框架(MOFs)材料具有良好的孔结构、较大的比表面积等特性,在气体储存、化学催化以及药物传输等方面显示出良好的应用前景[3-4].本研究利用NH2-MIL-101(Fe)作为催化剂,发现它能显著催化H2O2氧化TMB,产生蓝色反应,使体系的吸光度显著增加,表现出过氧化物模拟酶特性,而且在较宽的温度(4~80 ℃)及pH值(2~10)范围内保持其模拟酶活性,据此,结合葡萄糖氧化酶,建立了测定血清中葡萄糖的新方法.
全文HTML
-
UV-2450型紫外-可见分光光度计(岛津,苏州). 3,3,5,5-四甲基联苯胺(TMB)和葡萄糖氧化酶(GOx)均购买于Sigma-Aldrich上海有限公司. FeCl3·6H2O、N,N-二甲基甲酰胺(DMF)、H2O2、盐酸、氢氧化钠、NaAc、HA、无水乙醇、葡萄糖、果糖、乳糖和麦芽糖均购自重庆泰兴化学试剂公司. 2-氨基对苯二甲酸(NH2-H2BDC)购自TCI化成工业发展有限公司(上海).玻璃仪器用体积分数为10%的硝酸溶液浸泡24 h,使用前用超纯水清洗干净.
-
根据文献方法制备NH2-MIL-101(Fe) [5],将0.225 g NH2-H2BDC与0.675 g FeCl3·6H2O分别溶于一定量的DMF中,搅拌至完全溶解后,放入反应釜中加热至110 ℃,反应24 h,然后冷却至室温,并用DMF、乙醇交替洗涤,最后在60 ℃下真空干燥,即得NH2-MIL-101(Fe).
-
取0.5 mL质量浓度为20 mg/L的NH2-MIL-101(Fe)、0.25 mL浓度为1 mmol/L的TMB溶液及0.25 mL不同浓度的H2O2加入浓度为0.2 mol/L的NaAc缓冲溶液(pH=4)中,定容至5 mL后,在30 ℃水浴中孵育20 min,冷却至室温后,用紫外-可见分光光度计进行测定,记录652 nm处的吸光度.以吸光度变化值ΔA=A-A0进行定量(A0和A分别为未加入和加入H2O2时的吸光度).
葡萄糖测定:在系列比色管中依次加入0.1 mL质量浓度为1 g/L的GOx、0.5 mL醋酸钠缓冲液(pH=7)及0.1 mL不同浓度的葡萄糖溶液,在37 ℃水浴条件下孵育30 min后,按照前面检测H2O2的过程进行比色测定.
-
将NH2-MIL-101(Fe)在不同温度(4,8,15,20,25,30,40,50,60,70,80 ℃)和pH值(2,3,4,5,6,7,8,9,10)条件下孵育2 h后,按照1.3节的条件进行测定,考察NH2-MIL-101(Fe)作为模拟酶的耐受性.
1.1. 仪器及试剂
1.2. NH2-MIL-101(Fe)的合成
1.3. 测定方法
1.4. NH2-MIL-101(Fe)的耐受性
-
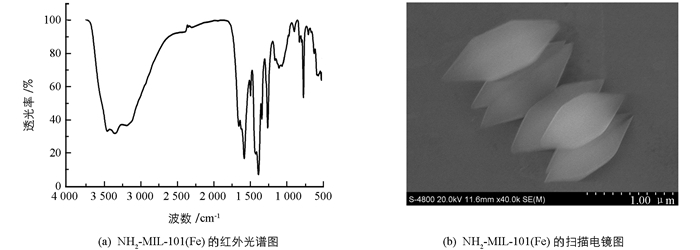
利用水热法合成了NH2-MIL-101(Fe),图 1(a)是其红外光谱图,其中3458 cm-1及3352 cm-1是—NH2的对称和不对称的伸缩振动峰[6],1384 cm-1处是C—N的振动峰,这主要是芳香基团引起的,与文献[6]结果相符.从扫描电镜图(图 1(b))可以看出,合成的NH2-MIL-101(Fe)呈现灯笼状,且尺寸大小均匀.
-
研究了NH2-MIL-101(Fe)催化H2O2氧化TMB的反应,结果见图 2(a).由图 2(a)可见,与TMB+ NH2-MIL-101(Fe)体系比较,TMB+ NH2-MIL-101(Fe)+H2O2体系在652 nm处的吸收峰明显增加,表明NH2-MIL-101(Fe)具有过氧化物模拟酶活性.
探讨了pH值、温度、材料质量浓度等条件对其活性的影响(图 2(b)及图 2(c)).可以看出,当pH值为4时其模拟酶活性达到最大值(图 2(b)).当反应温度从15 ℃增加到30 ℃时,酶活性迅速增加至最大值;当反应温度高于30 ℃后,酶活性降低(图 2(c)).故,选择30 ℃作为后续实验的最佳温度.实验了材料质量浓度的影响,当材料质量浓度达到20 mg/L时,模拟酶的活性增加趋势缓慢(图 2(c)).故,选择20 mg/L进行后续实验.在上述优化条件下,考察了过氧化氢浓度对显色反应的影响,发现652 nm处的吸光度随着过氧化氢浓度增加逐渐增大(图 2(d)).
-
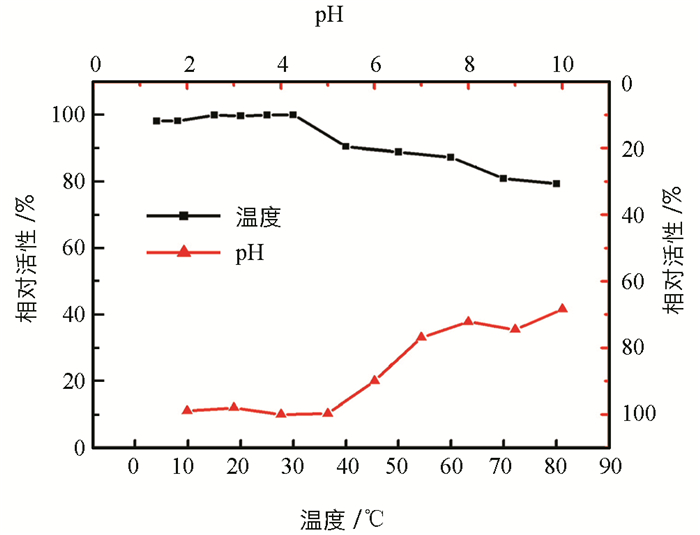
为了探讨NH2-MIL-101(Fe)的耐受性,将NH2-MIL-101(Fe)分别在不同温度(4~80 ℃)及pH值(2~10)条件下孵育2 h,再进行活性测定.从图 3可以看出,当温度在4~30 ℃范围内时,模拟酶活性基本保持不变,当孵育温度超过30 ℃时,模拟酶活性开始下降,但即使孵育温度达到80 ℃,材料的模拟酶活性仍然可以保持80%左右,表明NH2-MIL-101(Fe)耐热性较好. NH2-MIL-101(Fe)也表现出良好的耐受pH值的能力,如用pH=10的溶液处理后,其活性也可保持70%左右.研究结果表明NH2-MIL-101(Fe)具有良好的温度及pH值耐受性.
-
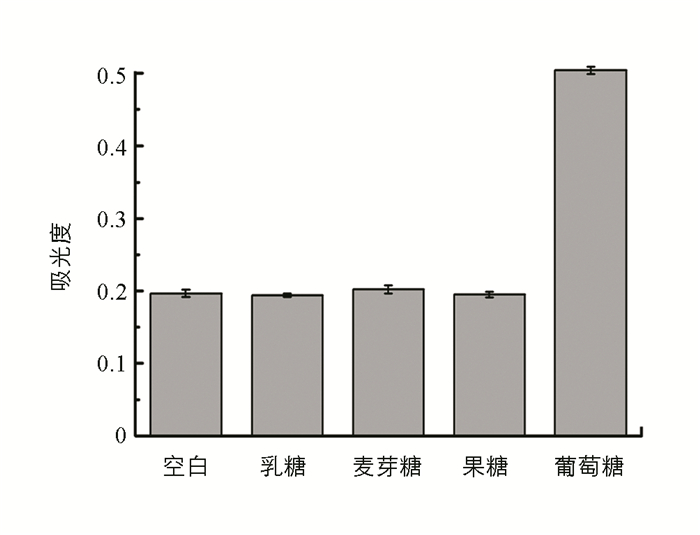
基于TMB的显色反应与过氧化氢浓度相关的现象,结合葡萄糖氧化酶,建立了测定葡萄糖的方法.在上述优化条件下,葡萄糖浓度在0.75~50 μmol/L范围内与吸光度ΔA呈良好的线性关系,线性方程为ΔA=0.006c+0.005(r2=0.9940,n=9),对葡萄糖的检出限为0.75 μmol/L.考察了葡萄糖类似物,如果糖、麦芽糖、乳糖对测定0.1 mmol/L葡萄糖的干扰,结果表明,在5%的误差允许范围内,相同浓度的果糖、麦芽糖、乳糖均不干扰葡萄糖的测定,表明基于NH2-MIL-101(Fe)测定葡萄糖的方法有良好的选择性(图 4).
-
将本法用于人血清中葡萄糖的检测,3份血清样品来自重庆市第九人民医院.测定前,血清样品用30 kDa Amicon Cell在3 000 r/min的转速下离心30 min,将滤液稀释20倍后,按照1.3节的方法测定葡萄糖,结果见表 1.由表 1可见,用本文的方法测得的人血清葡萄糖的结果与GOD-PAP法测定值相吻合,说明本法的准确度高,可用于实际样品分析.



 下载:
下载: