-
氯消毒是一种十分常见的消毒方式,也是目前世界上使用最广泛的一种饮用水消毒方式[1-3],美国自来水厂中约有94.5%采用氯消毒[4],我国约有99.5%以上水厂采用氯消毒[5].但是,氯在消毒过程中会与水中的天然有机物反应产生消毒副产物(disinfection by-products,DBPs),目前在饮用水中检出的消毒副产物中,卤乙酸(haloacetic acids,HAAs)和三卤甲烷(trihalomethanes,THMs)的含量最高[6-9],大约占总卤代DBPs的25%[10].许多研究人员通过实验证明,DBPs对动物和人类会造成不同程度的损害,严重的话还有可能致癌[2-3, 6].动物实验表明,4种THMs对大鼠均具有致癌性,三氯甲烷(trichloromethane,TCM)、溴仿(tribromethane,TBM)、二溴一氯甲烷(dibromochloromethane,DBCM)和一溴二氯甲烷(Bromodichloromethane,BDCM)分别能够引起大鼠的肝肿瘤、肠肿瘤、和肾肿瘤[1-2, 11];DBCM诱导染色体变异或姐妹染色体互换,TBM诱导姐妹染色体互换[12].体外实验表明,5种HAAs都可以导致细菌发生突变并破坏哺乳动物细胞中的DNA[13-16];在摄入途径暴露中,二溴乙酸(dibromoacetic acid,DBAA)、二氯乙酸(dichloroacetic acid,DCAA)和三氯乙酸(trichloroacetic acid,TCAA)会导致肿瘤产生,一氯乙酸(Monochloroacetic acid,MCAA)、一溴乙酸(Monobromoacetic Acid,MBAA)也有一定的细胞毒性[17].在卤代消毒副产物中(chlorinated disinfection by-products, Cl-DBPs),氯代三卤甲烷(Cl-THMs)和氯代卤乙酸(Cl-HAAs)的含量是最高的,人们对于这两种物质的关注程度也是最高的[9],也对其进行了比较充分的研究[18].但是,研究发现,相对于Cl-DBPs,虽然溴代消毒副产物(brominated disinfection byproducts,Br-DBPs)的浓度不高,但毒性更强[1, 7, 9, 19],有研究显示,后者的细胞毒性和基因毒性指数比前者高出近千倍[20]. THMs对慢性CHO细胞的细胞毒性的排序为TBM>DBCM>TCM>BDCM,致诱变潜能的排序是TBM>BDCM>DBCM[21]. 5种HAAs的细胞毒性顺序为MBAA>DBAA>MCAA>DCAA>TCAA,遗传毒性顺序为MBAA>MCAA>DBAA>TBA[22].因而Br-DBPs也引起了越来越多的关注[1, 9, 22-23].
溴离子大多作为一种阴离子广泛存在于各类水体中,主要来自海水入侵、工业和油田废水[1, 6, 11].世界范围内各类引用水源中溴离子质量浓度为2~4 mg·L-1[24].根据对我国不同地区13处饮用水水源水质的调查,溴离子质量浓度为10~249 μg·L-1,其中有8处高于50 μg·L-1[3].在对饮用水进行预氯化和消毒时,次氯酸(hypochlorous acid,HOCl)与溴离子迅速发生氧化反应,生成次溴酸(hypobromous acid,HOBr),水中的一些天然物质与HOBr也会发生反应,生成Br-DBPs,这一过程改变了水中DBPs的组成及比例[6, 11, 25-27].根据动力学研究的结果显示,相对于HOCl与有机物之间的反应来说,HOBr的反应速度要更快[3, 6-9],因此,溴离子的质量浓度虽然相对于氯的质量浓度要低很多,但是依然优先生成Br-DBPs.在低质量浓度环境下,溴离子的影响是不可忽视的,且随着溴离子质量浓度的逐渐升高,DBPs会逐渐由氯代产物逐渐演变为氯代溴代混合产物,反应完成之后,就会完全变为溴代产物[8-9].所以,有必要对于Br-DBPs的控制投入更多的研究.
目前关于消毒副产物的研究主要是THMs和HAAs中受管制的几种,而且这几类在常规水处理工艺出水中的浓度最高,并被列入了容易致癌的清单.以往的研究大都是在高浓度溴离子条件下针对氯消毒过程所产生的Br-DBPs[1, 4],其浓度远高于自然水体中的值,结果不能表达真实过程.因此,本文就受管制的4种THMs和5种HAAs进行研究,主要分析低质量浓度溴离子变化对水处理过程中产生的THMs,HAAs质量浓度及其对溴在二者中分配的影响,以期为控制水处理过程中生成的Br-DBPs提供依据,比高质量浓度溴离子的研究具有更强的现实意义.
全文HTML
-
本次实验的水样为天津市某给水厂预加氯之前的原水,在2014年5月对水进行4次取样,每周取样一次并进行检测.常规水质指标见表 1.由表 1可以看出,实验期间原水中氨氮的质量浓度为0.16~0.23 mg·L-1,浓度较低,因此其对氯与DBPs反应产生的影响可以忽略不计[28].
-
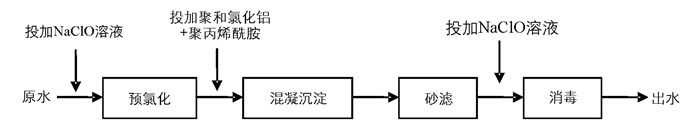
根据给水厂参数,对给水工艺进行实验室模拟,具体可见图 1.
总流程为:在预氯化工艺中,有效投氯量(以次氯酸钠计)为1.2 mg·L-1,接触反应3 min.用程控混凝实验搅拌仪进行混凝实验,聚合氯化铝(30 mg·L-1)作为混凝剂,聚丙烯酰胺(0.15 mg·L-1)作为助凝剂.投加完成之后,以300 r·min-1的速度搅拌30 s,速度梯度(Gradient of velocity,G)为420 s-1.絮凝过程可以分为两个阶段进行,第一阶段以150 r·min-1的速度搅拌3 min,G=165 s-1,第二阶段以30 r·min-1的速度搅拌10 min,G=32 s-1,搅拌完成之后,静置30 min即可进行过滤.选用滤柱高度为1 000 mm、直径为50 mm的有机玻璃滤柱过滤,滤料选用单层石英砂,层厚600 mm,粒径0.8~1.2 mm,承托层则铺设200 mm厚的卵石.采用次氯酸钠进行消毒,有效投氯量1.5 mg·L-1,接触反应30 min.
-
实验原水等体积分成4份,检测所取原水中的溴离子质量浓度值,然后分别向原水中投加不同量的溴化钠溶液,使得原水中溴离子的质量浓度分别达到50,100,150和200 μg·L-1.投加溴离子后的2组原水分别经过图 1所示工艺流程,分别取各单元工艺的出水,并进行各个水质指标的检测,24 h内检测完成.
-
本次需要检测的项目主要有4种受管制的THMs(包括TCM,BDCM,DMCM,TBM)、受管制的5种HAAs(包括MCAA,DCAA,TCAA,MBAA,DBAA)和溴离子.
采用配备μECD检测器的Angilent 6890N气相色谱仪分别对THMs和HAAs进行检测,所采用色谱柱均为毛细管色谱柱HP-5(30.0×0.25 mm×0.25 μm). THMs的测定采用改良的EPA标准方法551.1[29]:用液液萃取富集水样,用气相色谱对THMs定量检测.升温程序为30 ℃保持2 min,以5 ℃·min-1的速率升温至70 ℃. HAAs的测定采用改良的EPA方法552.3[30]:萃取剂为甲基叔丁基醚,酸性条件下,升温程序为35 ℃保持7 min,以5 ℃·min-1的速率升温至70 ℃,之后以3 ℃·min-1的速率升温至250 ℃,保持5 min.
采用配备电导检测器的离子色谱仪Dinoex ICS-1500对溴离子进行检测,所用色谱柱为AS23分析柱+AG23保护柱.待测水样首先需要过滤,滤膜选择0.45 μm厚,然后将1 mL过滤之后的水样滴加到离子色谱进样口中,进行离子检测.
-
为确保质量,实验过程中每批原水样平均分成2份做平行样分析,并且以实验用水作为空白对照,只接受相对标准偏差低于10%的数据,不符合此要求的重新进行分析.
三卤甲烷和卤乙酸的测定采用外标法.使用的标准参考物质来自于国家标准物质研究中心,并且标准参考物质的回收率均在90%以上.标准参考物质不断稀释直到峰值信噪比达到3,选择与此对应的0.01 μg·L-1作为4种THMs和5种HAAs的检测限.
1.1. 原水水质
1.2. 处理工艺和运行参数
1.3. 实验方法
1.4. 检测指标及方法
1.5. 质保措施
-
本实验针对不同溴离子质量浓度时4种THMs和5种HAAs在不同水处理工艺中的变化进行了研究分析.根据检测结果,4次采样样品的质量浓度变化并不是很大,所以采取均值分析的方法.经检测,溴离子平均质量浓度为50 μg·L-1. THMs平均质量浓度为11.68 μg·L-1. 4种THMs中,TBM未检测出,TCM,BDCM,DBCM的平均质量浓度分别为8.27,2.29和1.12 μg·L-1. HAAs的平均质量浓度为11.70 μg·L-1,MCAA,DCAA,TCAA,MBAA,DBAA的平均质量浓度分别为0.70,4.22,4.62,0.95和1.21 μg·L-1.
-
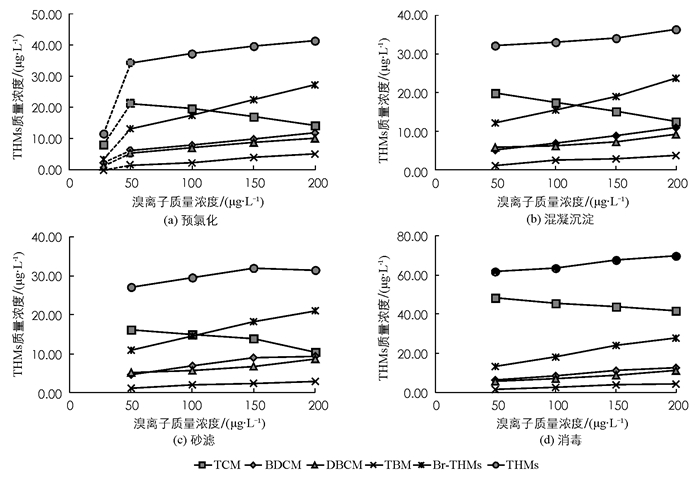
图 2反映了预氯化之后4种THMs质量浓度随溴离子质量浓度的具体变化.总体规律是:在各工艺中Br-THMs和THMs质量浓度随着溴离子质量浓度的增加呈现不同程度的上升,TCM则相反,呈现下降趋势.
在预氯化出水中(图 2a),各THMs都比原水中的质量浓度有不同程度的升高,这是由于NaClO的大量投加,使得水中HOCl质量浓度升高,其强氧化性有助于Cl-THMs和Br-THMs的生成.随着溴离子质量浓度的不断提高,Br-THMs和Cl-THMs质量浓度分别有不同程度的上升和下降,产生这种现象的原因是,HOCl将溴离子氧化,生成了HOBr,相较于HClO,HOBr具有更快的反应速度和更强的取代性[2, 7, 31],因此Br-DBPs能够先于Cl-DBPs生成.又由于有效投氯量一定,且预氯化反应过程迅速,在溴离子质量浓度不断增加的情况下,水中生成的Br-THMs质量浓度也在不断增加,而水中的有效氯不断降低,TCM的质量浓度也会随之下降;而由于HOBr更易与有机物反应[7],使得Br-THMs的质量浓度的升高高于TCM质量浓度的下降,最终表现为总THMs的质量浓度升高.变化规律与梅红等[27]的研究结果类似.
混凝沉淀出水(图 2b)中,各THMs的质量浓度均低于预氯化(图 2a)出水中THMs的质量浓度,这主要是因为:一方面,混凝工艺降低了卤素与天然有机物之间的反应速率[7],在一定反应时间内,使得THMs的生成量降低;另一方面,TCM是THMs的主要组分,其为疏水性物质,在混凝沉淀过程中易于被吸附在絮凝体上而去除[32].
混凝沉淀出水(图 2c)经过砂滤之后,虽然有极少数BDCM和TBM的质量浓度略有升高,但由于TCM质量浓度下降幅度较大,所以THMs的质量浓度有一定程度的下降,主要是因为THMs为疏水性物质,随着混凝沉淀,也会有一部分的絮凝体被留在滤柱中;且石英砂的表面也带有一定的电荷,能够吸附部分的THMs,也能达到一定的THMs去除作用[33],这与之前的研究结果一致[32].
对比图 2c和图 2d可以得知,比之砂滤工艺,在消毒过程中因为氯的投加,THMs的质量浓度大幅度提高,最高达69.68 μg·L-1,此时,水中溴离子的质量浓度为200 μg·L-1,说明氯离子和溴离子质量浓度的适度增加会提高THMs的生成量,这与其他研究的结果是一致的[23].分别考察4种THMs的变化可以看出,当溴离子质量浓度分别为50,100,150,200 μg·L-1时,测得水中TCM质量浓度有了不同程度的上升,分别上升了32.15,30.48,28.59,31.38 μg·L-1.而BDCM、DBCM和TBM在加氯之后的质量浓度与砂滤出水相比,上升幅度并不高,这主要是因为本研究设定的溴离子质量浓度变化范围比较低,Br-DBPs的生成受到了溴离子质量浓度的限制.这与高浓度溴离子条件下的结论是有很大区别的[34].
另外,由图 2可知,DBPs中TCM的质量浓度始终高于3种Br-THMs的质量浓度.造成这种现象主要是本实验旨在研究低质量浓度溴离子变化对于消毒副产物的影响,所以设定的溴离子质量浓度较低,一定程度上限制了Br-THMs的生成.有研究表明,当水中溴离子的质量浓度达到一定值时,优势DBPs就会发生变化,王昊宇[34]通过研究证明,溴离子质量浓度在200~1 000 μg·L-1变化,当溴离子质量浓度增加到500 μg·L-1时,DBCM的生成质量浓度最高,而溴离子的质量浓度为1 000 μg·L-1时,生成质量浓度最高的物质为TBM.
-
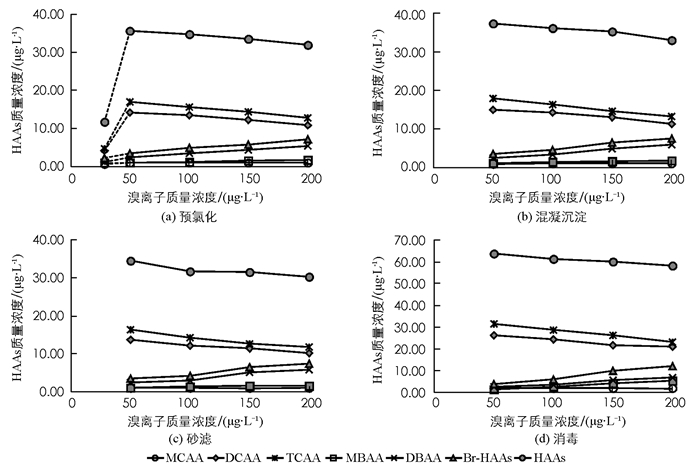
图 3反映了各工艺出水中5种HAAs质量浓度随溴离子质量浓度的具体变化.总体规律是:随着溴离子质量浓度的提高,各工艺出水中的DBAA,MBAA质量浓度逐渐上升,MCAA质量浓度基本保持不变,DCAA,TCAA逐渐下降,且各工艺出水中的DCAA和TCAA质量浓度均远高于其余几种HAAs的质量浓度,占据绝对的优势,这与Zhang等[3]、Bougeard等[19]的研究结果一致. 5种HAAs的总量呈现下降趋势,说明这5种HAAs在设定的溴离子质量浓度范围内并不是优势物质.造成这种现象主要是因为HOBr比HOCl具有更强的取代性[8, 27],使Br-HAAs生成的优先级高于Cl-HAAs,所以表现为随着溴离子质量浓度的不断升高,Br-HAAs的质量浓度有不同程度的提高,Cl-DBPs的质量浓度下降或者基本保持不变.
溴离子质量浓度为50 μg·L-1时,也就是未额外加溴离子时,随着预氯化过程中NaClO的大量投加,DBAA,MBAA,DCAA,TCAA质量浓度都有不同程度的提高(图 3a),说明由于HClO的强氧化性,其质量浓度的提高有利于DBPs的生成.
当溴离子的质量浓度分别为50,100,150,200 μ g ·L-1时,混凝沉淀工艺出水中HAAs的质量浓度分别为37.33,36.12,35.23,33.07 μg·L-1(图 3b),其含量均要高出预氯化单元出水中HAAs的质量浓度.这主要是由于HAAs的亲水性,混凝沉淀工艺对其基本没有去除作用[34]且在此单元内还生成了一部分的HAAs,另外,由于预氯化单元的接触反应时间只有3 min,所以还存在残留的氯,在混凝沉淀工艺中,残留下来的氯会继续发生反应,这也是HAAs的质量浓度升高的重要因素.
对比图 3b和图 3c可知,当水中溴离子的质量浓度分别为50,100,150,200 μg·L-1时,进行砂滤后,水中HAAs质量浓度均有所下降,下降值分别为2.83,4.40,3.67,2.85 μg·L-1,说明砂滤工艺能去除一定的HAAs,这与文献中的研究结果一致[34].分别考察图 3c中5种HAAs的变化可以看出,与图 3b相比,砂滤工艺出水中MCAA和MBAA的质量浓度几乎不变,其他3种HAAs的质量浓度均有不同程度的下降,且DBAA的下降程度小于DCAA和TCAA.
对比图 3c和图 3d可以得知,相较于砂滤工艺出水,消毒过程投加氯使得HAAs的质量浓度大幅度提升,在溴离子质量浓度为50 μg·L-1时,HAAs质量浓度达到最高(63.80 μg·L-1).对5种不同的HAAs变化进行考察,发现水中溴离子质量浓度为50 μg·L-1时,DCAA和TCAA的质量浓度提升最大,分别达26.15和31.59 μg·L-1;MCAA的质量浓度上升都在1 μg·L-1左右;MBAA的质量浓度提升明显,出水最大质量浓度达5.35 μg·L-1;DBAA的质量浓度较之前并没有大幅度的提升,出水最大质量浓度为6.89 μg·L-1.这是因为其中大部分的溴离子在前3步处理过程中被消耗,使得在消毒过程时,可发生取代反应的溴不足,导致此过程中DBAA的生成质量浓度低于MBAA.
-
人们通常运用溴结合因子(Bromide Incorporation Factor,BIF)来表示溴对DBPs的贡献程度[35].本研究为了进一步分析Br-THMs在总THMs和Br-HAAs在总HAAs中所占的比例引进溴结合因子. THMs和HAAs的溴结合因子分别用n(THMs)和n(HAAs)表示,其计算公式如下所示:
式中,THMs表示的是THMs类物质的总和(μmol·L-1),HAAs表示的是5种不同HAAs物质的和(μmol·L-1).
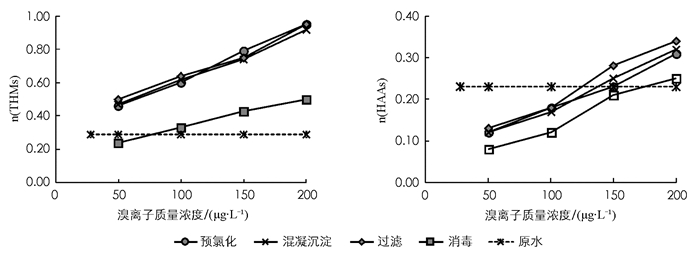
THMs和HAAs的溴结合因子范围分别在(0,3)和(0,2),值越大表示溴对THMs或HAAs的贡献越大,反映出Br-DBPs所占比例越大[35].本文THMs和HAAs的溴结合因子在水处理工艺中的变化如图 4所示.
根据图 4可知,当溴离子的质量浓度从50 μg·L-1增加到200 μg·L-1,各工艺中THMs和HAAs的溴结合因子均不断升高,这表明在一定质量浓度范围内,溴离子质量浓度的增加更有利于Br-DBPs的生成,这与前人的研究结果一致[27, 31, 36].但是,HAAs的溴结合因子始终小于THMs的溴结合因子,且与THMs相比,溴离子质量浓度对n(HAAs)产生变化的影响程度较小,这就说明相对于THMs来说,HAAs各组分分配比例受溴离子质量浓度影响更小,这与孙媛媛等[37]、Ates等[31]的研究结果一致,这主要是因为溴更易于与NOM反应生成Br-THMs,且THMs前体物比HAAs的前体物对于溴离子质量浓度的变化更敏感[37].
进一步分析各工艺中THMs溴结合因子的变化可以发现,当溴离子质量浓度为50 μg·L-1时,经过预氯化之后THMs的溴结合因子由原水中的0.29上升到了0.46,HAAs的溴结合因子由原水中的0.23下降到了0.12.而混凝沉淀和砂滤出水中的THMs和HAAs的溴结合因子与预氯化出水相比基本保持不变,最后经过消毒工艺之后THMs和HAAs的溴结合因子分别下降到了0.24和0.08.由此可见,在本研究设定的溴离子质量浓度变化范围内,只有预氯化和消毒工艺可以改变水中Cl-DBPs和Br-DBPs的生成比例.出现上述现象是因为:当氯的投加量一定时,溴离子质量浓度的增加,有利于Br-DBPs的生成,Br-DBPs占DBPs的比例增加,表现为溴结合因子增大;当原水中溴离子质量浓度一定时,投氯量增加有利于Br-DBPs和Cl-DBPs的生成,当投加氯质量浓度较低时,生成DBPs的量一定,又由于Br-DBps优先生成,所以Br-DBps占DBPs的比重较大,表现为溴结合因子的提高,但在消毒过程中,又向水样中投加了氯,Br-DBPs和Cl-DBPs生成量均增加,而Br-DBPs的生成受到Br的限制,表现为溴结合因子降低.又由于Br-THMs优先于Br-HAAs生成,所以在氯离子质量浓度较低时,HAAs的溴结合因子降低.
-
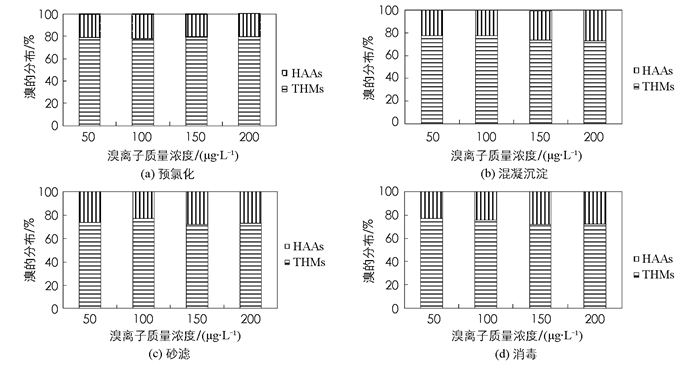
上文主要针对不同溴离子质量浓度时,考察不同水处理工艺出水中Br-THMs和Br-HAAs的质量浓度的变化,发现原水中溴离子的质量浓度发生变化时,出水中Br-DBPs的量和溴在DBPs(THMs和HAAs)中的比例都会随之变化.
由图 5可知,当水中溴离子质量浓度产生变化时,THMs和HAAs 2组物质中,溴的分配并没有产生很大的变化,这一结果说明,高质量浓度溴离子实验的结果与本次试验的结果存在很大区别[36].溴在THMs中的比例为72%~79%,在HAAs中的比例为21%~28%.可见,溴离子在THMs中的比例远高于HAAs,这主要是因为溴离子与NOM反应生成Br-THMs的速率高于Br-HAAs,Br-THMs优先生成[37].如图 5a,在预氯化工艺中,当溴离子质量浓度发生变化时,THMs和HAAs中溴元素的比例基本保持在80%和20%,虽然有一定的浮动,但变化并不明显. 图 5b显示的是混凝沉淀工艺情况,当溴离子质量浓度在50~200 μg·L-1变化时,溴在HAAs中的比例由23%增加到27%.产生这些现象是因为,当水中溴过多时,余溴会与其他有机物发生化学反应,生成Br-HAAs和Br-THMs,且混凝工艺中Br-HAAs的去除不明显,使得HAAs中Br的比例升高. 图 5c显示的是砂滤工艺中的现象,在HAAs中,溴的比例基本保持在27%,这与混凝沉淀工艺相比,有一定上升,主要原因是Br-THMs在砂滤工艺中的去除率更高. 图 6d表示的是在消毒工艺中,当水中溴离子的质量浓度增加时,溴在THMs中的比例逐渐降低,由76%下降至71%.这主要是因为,在消毒工艺出水中,溴离子和氯离子质量浓度都较高,且反应时间较长(30 min),MBAA生成量大幅度提高,最终表现为Br-HAAs比例的提高,而在预氯化出水中(图 5a)由于反应时间短(3 min),所以规律并不明显.其根本原因是随着反应进行,THMs的前体有机物下降,使得溴与HAAs的反应速度加快,最终导致Br-HAAs的比例上升.
2.1. 溴离子浓度对不同水处理工艺中THMs生成的影响
2.2. 溴离子质量浓度对不同水处理工艺中HAAs生成的影响
2.3. 溴离子质量浓度对水处理工艺中THMs生成比例的影响
2.4. 溴离子质量浓度对溴在THMs和HAAs中分配的影响
-
(1) 在水处理工艺中,当溴离子质量浓度范围为50~200 μg·L-1时,随着溴离子质量浓度的逐渐增加,Br-THMs和总THMs的质量浓度也会随之增加,Cl-THMs则呈下降趋势,n(THMs)升高.
(2) 在水处理工艺中,溴离子质量浓度范围为50~200 μg·L-1时,随着溴离子质量浓度的增加,Br-HAAs的生成质量浓度逐步增加,5种HAAs的生成质量浓度逐步下降,DBAA的生成质量浓度逐步增加,而DCAA和TCAA的质量浓度会随之下降,n(HAAs)升高.
(3) 在预氯化、混凝沉淀、砂滤和消毒过程中,只有预氯化和消毒工艺能明显改变水中Br-DBPs和Cl-DBPs的比例.
(4) 当原水溴离子质量浓度为50~200 μg·L-1时,溴离子质量浓度的变化对溴在THMs和HAAs中的分配比例有一定的影响,但影响不大.



 下载:
下载: