-
开放科学(资源服务)标识码(OSID):

-
高镍三元材料为正极的锂离子电池具有能量密度高、环境友好等优点,有着广泛的应用前景和巨大潜在的经济效益[1],适合大量投放市场进行规模生产,但其自身的容量衰减和安全问题不容忽视,高镍三元电池随着高镍正极中的Ni含量提升,电池的容量衰减快速加剧,严重影响电池的应用. 一般而言,锂离子电池中所用的石墨或硅基负极材料的容量设定通常高于正极材料,因此全电池的容量主要取决于正极材料的总容量. 而高镍三元正极在制备和充放电循环中常伴随着阳离子混排、表面反应、材料中过渡金属元素的迁移与溶解析出、氧空位的产生、相变和微裂纹的产生等问题,促使电池的容量快速衰减.
除了正极材料以外,作为构成电池的主要组成成分的负极和电解液也对锂离子电池容量衰减有影响. 负极材料的自身结构、体积膨胀的大小、表面SEI膜的完整性等也与电池的循环性能息息相关. 作为与正、负极相接触的电解液,其自身的化学稳定、电化学稳定性及与电极相容性和副反应也影响着电池容量衰减的程度.
综上,本研究从高镍三元锂离子电池重要组成成分:正极、负极和电解液,分别探讨其影响全电池性能衰减的机理,以期通过探究其深层次机理,找到提升高镍三元正极材料锂离子电池循环稳定性的突破口,为开发综合性能佳且容量衰减程度小高镍三元锂离子电池奠定理论基础.
全文HTML
-
在锂离子电池的正极材料开发过程中,层状LiCoO2因其工作电压高、充放电电压平稳、理论比容量高(约为274mAh/g)、锂离子的扩散系数可达10-9~10-7cm2/s等优点,是最早被商业应用的正极材料. 但在锂离子电池深度充电时,Li离子在LiCoO2中的过量脱出会导致Co的不可逆迁移,诱发结构发生不可逆变化. 此外,脱锂程度较高时,高氧化态的Co会与电解液发生副反应. 因此层状LiCoO2正极材料实际比容量仅为理论比容量的50%~60%,高电压下循环不可逆的容量损失严重. 同时,Co毒性较大,还会带来相应的安全隐患,另外,Co的价格较为昂贵且资源较少,进一步限制了LiCoO2在其他领域的商业应用.
LiNiO2正极材料与LiCoO2均具有相同的α-NaFeO2层状结构[2],如图 1所示,且Ni资源相对丰富,与Co同为第一过渡系元素,因此对LiNiO2的研究逐渐得到了人们的关注. 层状LiNiO2正极材料理论比容量接近280 mAh/g,实际容量可达理论比容量的70%. 相较于Co而言,其工作电压、实际比容量均有所提升,且镍盐相对安全且环境友好,使其成为更具潜力的锂离子电池正极材料. 然而,LiNiO2正极材料在循环过程中发生多结构转变,严重影响其循环稳定性. 此外,制备纯相层状LiNiO2也具有一定难度,其放电能力很大程度上取决于Li/Ni比、阳离子混排程度和Ni的氧化态[3].
随着对锂离子电池正极材料的不断研究,科研人员亦将注意力转移到与Co同周期的过渡系金属元素Mn. 研究发现以Mn代替LiCoO2和LiNiO2中的过渡金属会产生两种常见形式,分别是具有层状结构的LiMnO2和具有尖晶石结构的LiMn2O4. 其中,层状结构的LiMnO2热力学不稳定,易发生相变而导致电池容量的快速衰减. 而尖晶石结构的LiMn2O4较为稳定,成本低、安全性较好,实际容量与理论容量相近,但其理论容量较低,在高温下容量衰减过快是限制其发展的主要原因.
上述含单一过渡金属元素的正极材料各有优劣,科研人员逐渐开始尝试掺杂策略改性层状结构正极材料,以Ni,Co,Mn和Al等元素部分替代单一过渡金属元素,旨在通过插入元素的优势优化材料性能,如LiNixCo1-xO2[4],LiCoxMn1-xO2[5],LiNixAl1-xO2[6](0<x<1)等. Yoshio等人[3]用Mn部分替代LiNiO2中的Ni,发现当Li含量在0.99~1.10范围内,其放电容量不受Li/(Ni+Mn)比的影响,也一定程度上解决了容量衰减的问题. 采用这一掺杂策略设计合成出LixNiyCozMn1-y-zO2(NCM)和LixNiyCozAl1-y-zO2(NCA)这两种材料性能更为优越的三元正极材料. Yoshio等人[7]在LiNi0.8Mn0.2O2中继续掺杂Co得到LiNi0.8-yMn0.2CoyO2(0<y<0.8),发现Co的掺杂可进一步加速过渡金属离子的氧化,抑制部分阳离子的混排.
随着对电池需求的日益提升,能量密度成为限制锂离子电池发展的主要因素. 人们发现在NCM或NCA的基础上,将Ni含量提升至过渡金属的60%以上可大幅提升电池的能量密度,即高镍三元正极材料. 由于其具有高可逆容量(180~220 mAh/g)、高工作电压、低成本,被视为最有发展潜力的下一代高能量密度锂离子电池.
-
高镍三元材料被认为是在LiCoO2的基础上进行Ni、Mn或Al的元素掺杂改性,结构式为LiNixCoyM1-x-yO2,其中x≥0.6,0<y<0.4(x,y均为摩尔分数),M为Mn或Al元素. 通常会以Ni,Co,Mn/Al的首字母进行简单命名,如M为Mn即命名为NCM,M为Al即命名为NCA. 其结构保持了LiCoO2的六方层状结构,空间点群为R3m. 在层状晶格中,锂离子主要占据3a位置,过渡金属离子占据3b位置,氧离子以立方密堆积的形式占据6c位置,整体构成MO6八面体结构(图 2).
-
Ni,Co,Mn 3种过渡金属元素在层状高镍三元材料中协同作用,研究表明Co和Al离子均呈+3价,Mn呈+4价,Ni则呈+2、+3混合价态(以+3价为主). 其中,Mn在反应前后价态不发生变化,一般被认为无电化学活性. 而Co与Ni的价态在充电过程中氧化成+4价,放电过程中回到初始价态,高镍三元正极中占主要成分的Ni被认为是主要的电化学活性物质.
高镍三元正极设计与合成中,适量使用Ni可提高正极材料的容量,过量则会加剧Li/Ni阳离子混排、与电解质的副反应、表面结构重建、微裂纹和降低热稳定性. Tsai等人[10]发现在首次充放电循环过后,高镍三元正极材料中Ni的氧化态发生了变化,Ni—O键的键长发生改变,揭示高镍三元正极首次不可逆容量是归因于在充放电过程中出现了惰性的Ni. Pan等人[11]采用NCM811,NCM721,NCM712,NCM622四种不同过渡金属元素含量的正极材料进行循环测试发现,50次循环后NCM622的容量保持率最高可达97.1%,XRD表征揭示子50次循环后,随着Ni含量的增高,NCM的层状结构无序性增加.
Co在高镍三元正极材料中能提高电池的倍率性能,抑制过渡金属的离子排列的无序性(阳离子混排),减少材料欧姆阻抗,提高材料电导率. 但Co含量过多也会导致电池的不可逆容量增多,同时因Co使用量增多,成本也随之加大. Wang等人[12]通过对比两种不同Co含量的NCM正极材料和无钴Li1.3Ni0.25Mn0.75O2.4正极材料发现,钴含量提升有利于材料形成更好的六方晶系结构、降低尖晶石相含量、阳离子混合程度和电荷转移阻抗,并提升材料放电比容量和倍率性能. Li等人[13]通过改变正极材料LiNi0.6CoxMn0.4-xO2中Co的含量,发现随着Co含量的增加,材料的首周放电比容量会提升,但XPS分析表明Co含量的增加会使Ni3+/Ni2+的比例增大,加剧材料充电/放电过程中的相变程度,从而导致材料循环稳定性下降.
Mn作为非电化学活性物质,在结构中起支撑作用,在锂离子在嵌入和脱出时维持材料层状晶体结构,提高了材料循环和热稳定性能. Zheng等人[14]对比多种高镍三元材料发现,具有最佳Mn含量(LiNi0.72Co0.10Mn0.18O2)的样品在循环过程中显示出显著增强的可循环性和电压衰减,材料的结构稳定性亦显著提升. 掺入富镍正极材料中的Mn离子,特别是富集于材料颗粒表面的Mn离子,可显著减少电极表面与电解质之间不可逆的副反应并改善表面结构稳定性. 另外,Hyung等人[15]通过实验还发现提高Mn的含量可以增强正极材料的热稳定性. 然而,层状三元中过量的Mn会使得层状结构中形成尖晶石结构杂相,破坏六方层状晶体结构,降低材料的电化学性能.
1.1. 高镍三元材料的开发历程
1.2. 层状高镍三元材料的组成及结构
1.3. 高镍三元材料中过渡金属的作用
-
在高镍三元正极材料的晶格中,锂离子主要占据3a位置,过渡金属离子无序占据3b位置. 其中,镍主要呈现的化合态是+3价,Ni3+的电子在eg轨道上是不均等排布,在Jahn-Teller效应的作用下,Ni3+有向Ni2+/4+歧化变化的趋势. 而锂离子的离子半径(0.076 nm)和二价镍离子的离子半径(0.069 nm)尺寸大小相近[16],所以Ni2+存在占据晶格中锂离子的位置的趋势,即发生阳离子混排现象(图 3),且阳离子的无序性会随着Ni2+的增多而增强. 这一现象在最初正极材料合成时便会存在. 除此之外,在充电过程中Li+从正极材料中脱出并嵌入负极的晶格之中,正极处于高电位的贫锂状态,此时氧层中O2-离子间的斥力便会推动部分Ni离子迁移至锂层稳定材料结构,占据锂空位,也可造成阳离子无序排布.
大量研究表明,阳离子混排所导致的结构缺陷会造成高镍三元材料锂离子电池的容量衰减. 其一,在锂离子电池中主要依靠Li+在正负极间的嵌入脱出来完成充放电工作,如果在充电时部分锂空位被Ni2+占据,而放电时Li+因Ni2+的存在阻碍锂离子正常嵌入锂层减少嵌脱锂位置,直接造成电池容量降低. 随着Ni2+占据程度逐渐加深,材料的结构也会相应发生不可逆变化,使其从六方层状结构变为立方岩盐相结构,逐步失去嵌入和脱嵌Li+的能力. 其二,当Ni2+替代Li+进入到层状结构中时,由于镍的正电性强于锂,故会使锂和氧的层间距减小,当Li+再嵌入回锂层时则需要更多的能量,相当于增加了Li+迁移所需的活化能,从而使其扩散电阻也在增加,导致电池的倍率性能受到损失. Wu等人[17]以NCM811为正极材料进行研究发现,Li+/Ni2+混排程度会随由XPS确定的Ni2+含量的增加而降低,循环稳定性和速率能力也会随Ni2+含量的增加而提高(图 3),电化学阻抗谱显示出电荷转移电阻在降低. 因此,适当增加Ni2+含量可以降低阳离子混排造成的无序性,同时增强电化学性能.
-
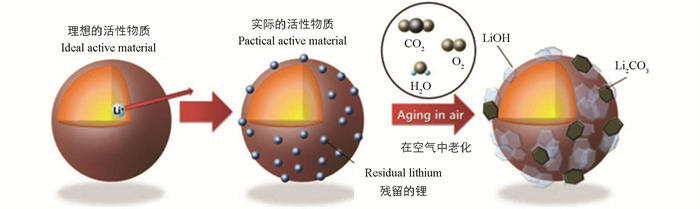
由于锂和镍的阳离子混排现象较为普遍,在合成高镍三元材料时往往会添加过量的锂来降低锂镍混排程度. 而锂盐极易与其他物质发生化学反应,例如,暴露在正极材料表面的残余锂盐可与空气中的水蒸气和二氧化碳发生化学反应,相应生成氢氧化锂和碳酸锂. 除了锂的额外损失会使电池容量受损外,氢氧化锂和碳酸锂的存在也会给电池的安全性能带来危害. 首先,氢氧化锂会升高电池内部的PH值,易与部分电解液反应成凝胶态,增加了浆料制备和电极装饰的难度,也增大了Li+传输的界面电阻. 其次,氢氧化锂与电解质盐(如LiPF6)反应生成腐蚀性较强的HF. 再者,碳酸锂的形成减少了颗粒之间的黏附作用,被认为是导致NCM材料产生严重降解的原因. 另外,充放电过程中碳酸锂易与电解液中的酸性物质发生副分解反应,产生的二氧化碳气体会导致电池存在气胀的情况,电池结构易受到损害;在高温下,碳酸锂稳定性降低,同样会分解产生气体导致气胀现象的发生.
此外,正极材料表面反应不均匀也会导致电池容量的快速衰减. 如图 4所示暴露在正极材料表面的锂脱出与从其内部脱出存在动力学差异,导致电子结构不均匀,会影响正极材料结构的稳定性.
-
在电池充、放电过程中,随着Li+的嵌入和脱出,部分过渡金属元素也会受到影响随之在材料内部发生迁移(图 5). 过渡金属元素的迁移会导致高镍材料的结构发生破坏[20],降低了材料的稳定性,同时部分物质会在正极表面溶出,增大了表面电阻,导致容量衰减.
在高镍三元正极材料所涉及的过渡金属元素中,Ni的迁移与溶解析出现象是最为严重的. 在电池充电时,Ni由于其具有低扩散势垒,会与Li+一同沿着Li+的扩散通道脱出,部分沉积在正极表面,部分进入电解液. 在Ni2+的迁移过程中Ni-O键发生断裂,降低了正极材料晶体的结晶度和稳定程度. Sun等人[21]通过共沉淀法成功合成了新型的具有浓度梯度的LiNi0.83Co0.07Mn0.10O2核壳结构正极材料,其中内核LiNi0.9Co0.05Mn0.05O2被完全封装,并形成了稳定的镍含量降低的浓度梯度外壳层. 经实验测定,富镍的内核LiNi0.9Co0.05Mn0.05O2中可释放出高容量,并通过具有LiNi0.68Co0.12Mn0.20O2的外壳表面组成的贫镍浓度梯度层实现了改善的热稳定性,与纯内核组成的高镍正极材料相比,浓度梯度材料具有良好的锂插层稳定性、高电池容量和热稳定性.
-
在高镍三元正极材料中,氧在晶体结构中密集堆积,其稳定性是通过和过渡金属元素成键来达到的. 晶格中的氧含量将直接影响到Ni和Co的核外电子数,决定了M-O的强度,以此显著作用于电池的放电比容量和循环稳定性[23]. 在过渡金属的溶解析出时,过渡金属与氧之间的键会发生断裂,造成晶格中的氧析出,导致氧缺失从而形成氧空位(图 6). 反之,由于氧空位而造成的层状结构缺陷,同样会增加正极材料的内阻,降低电化学性能.
氧空位的产生与阳离子混排亦存在关联. 阳离子混排形成的过程中,Ni3+有向Ni2+变化的趋势,正电荷总量会减少. 根据电荷守恒原理,作为正极材料中唯一显负价态的氧会随之受到影响,氧便会减少以此来平衡电荷,故氧会从晶体结构中脱出,形成氧空位.
此外,氧空位也被认为与正极材料深度脱锂有关. Hatsukade等人[24]通过DEMS-DEIRS实现同位素标记,发现高镍NCM在较高的脱锂程度下有利于形成氧空位,这与在高SOC下观察到的NCM结构不稳定相一致;且在高脱锂程度下,NCM表面的氧位点反应活性也会增强[25].
Min等人[26]发现利用Al掺杂这一方法,在NCM材料中可有效阻止氧空位的产生. 同时,经过对氧空位生成焓的计算和氧的电荷分析可知,无论结构是否有预先存在的缺陷或脱锂情况发生,在结构中掺杂Al可提升氧在晶体结构中的稳定性.
-
在以高镍三元材料为正极的锂离子电池充电时,正极表层脱锂状态会生成较多的Ni4+,Ni4+易与电解液反应还原为Ni2+,从而与氧结合生成立方岩盐相NiO. 除了新物质沉积在正极材料表面之外,在反应的过程中,也会促使材料体相从六方层状结构逐步向尖晶石结构转化,最终变为岩盐相(图 7),发生了不可逆的相变[28],恶化了电池的循环稳定性. 同时,生成的NiO是一种绝缘材料,影响Li+在材料中的离子传导,降低了Li+的扩散系数,增大了正极材料的表面电阻,降低了电池的容量[29]. Feng等人[30]在Ni0.8Co0.1Mn0.1(OH)2上涂布Mn为前驱体制备成具有自组装浓度梯度壳的的纳米管,发现这种制备方式可以有效抑制NCM811材料表面在循环过程中产生岩盐相,从而增强了正极材料的结构稳定性. 经电化学测试发现,在0.1C下,工作电压在2.8 V~4.45 V间循环100次后,其容量保持率可达92.2%. 此外,除了对容量衰减有改善效果外,还可以提高正极材料的热稳定性.
-
常见的高镍正极材料分为二次颗粒和单晶两种. 二次颗粒是由一次粒子紧密堆积形成的;高镍二次颗粒材料在充、放电时所对应的离子迁移和电子转移都是在二次颗粒内部完成[32]. 二次颗粒的内部一次粒子排列越紧密,正极材料的振实密度越高,亦意味着单位体积内所包含的正极材料含量越多,则电池的体积能量密度也得到增加. 在一般情况下,颗粒形状较为规则、分布均匀且晶体结晶程度高的二次粒子具有更高的振实密度. 姜涛等人[33]发现高振实密度不仅可以增大电池的能量密度,还可使正极材料具有较好的循环性能和倍率性能,综合提升正极材料的电化学性能.
对于高镍二次颗粒材料而言,在充、放电循环中,一次粒子的体积发生各向异性的变化并伴随着气体释放,从而导致颗粒间产生晶间微裂纹[34]. 在长期的电流作用下,还会导致一次粒子出现晶内裂纹,甚至颗粒破碎[35]. 上述微裂纹的形成会使得离子迁移和电子转移受到阻碍,使电阻增加,增大极化,从而造成电池的容量衰减. 图 8的扫描电镜图中展示了高镍NCM材料二次颗粒和一次颗粒经过100次循环后充电至4.3V的微裂纹情况.
对于高镍单晶材料而言,(003)晶内开裂是其主要的开裂机制. 可以观察到岩盐相沿(003)面发展形成岩盐片,岩盐片的破裂导致(003)开裂. 电化学活性层状相和非活性岩盐相之间的应力不连续性引起对岩盐片晶的循环机械冲击,从而导致岩盐晶格撕裂或破裂. 张应力集中在岩盐片晶的表面区域,导致裂纹尖端的成核和向内运动,造成一次粒子的(003)晶面产生裂纹,加速了结构退化并降低了正极的电化学性能[36]. Bi等人[37]以高性能单晶LiNi0.76Mn0.14Co0.1O2作为研究对象,观察到沿(003)平面存在很大程度的可逆平面滑动,而不可逆的滑动会产生较小的损伤,在长时间的循环累积作用下,出现了裂纹成核现象,导致了微裂纹的产生.
Yang等人[39]采用改性共沉淀法合成了具有独特孔壳结构的LiNi0.8Co0.1Al0.1O2,经原位扫描电镜和原位XRD测试发现这种结构有助于缓解在循环下产生的微应力,在1C速率下循环100次后,容量保持率可达90.33%. Su等人[40]将多孔结构引入到高镍三元正极材料颗粒中,即提供了一定的缓冲空间,可以达到减轻应力的目的;同时也抑制了相变的发生,提高了电池的循环稳定性. Yang等人[41]通过调整Al3+从体表面到内部的扩散路径,成功地在LiNi0.8Co0.15Al0.05O2中均匀掺杂Al3+,工艺思路如图 9所示. 这一策略中,铝与氧形成了具有高键能的Al-O键,提高了正极材料结构的稳定性,说明均匀的Al3+掺杂可以抑制二次晶粒破损.
另外,Liu等人[42]通过研究商用高镍NCA正极材料LiNi0.8Co0.15Al0.05O2的容量衰减机理发现,Li在正极材料中嵌入与脱出时会使晶格尺寸发生各向异性的变化,导致一次粒子的边界处产生晶间裂纹,降低二次粒子的离子和电子连通性,同时影响了材料的反应动力学,使反应进行不完全且速度迟缓. 晶间裂纹的形成也减少了相邻一次粒子间的接触面积,增加了离子和电子运输的阻抗,综上造成电池的容量损失.
除了与一次粒子体积各向异性变化有关,微裂纹的产生还与过渡金属元素离子的迁移有关. Kim等人[43]以LiNi0.6Co0.2Mn0.2O2为正极材料进行实验,发现过渡金属离子的迁移导致其发生相变,这种相变伴随着晶格常数和晶格体积的变化,也会导致氧从晶格中脱出,形成微裂纹. 同时也提出了一种改善微裂纹的方法,即提高正极材料中Co的含量并在其内部形成具有纳米级厚度的阳离子混合相,从而通过抑制材料的结构变化来减少微裂纹的形成. 伴随着晶间和晶内裂纹程度的不断增大,材料中会出现越来越多新的表面,形成新的反应活性位点,导致表面相变、副反应等情况发生,加速了电池老化,严重影响电化学性能[44-45].
微裂纹的产生也与充、放电循环中的放电深度(DOD)和温度有关. Watanabe等人[46]利用SEM和BET两种测试手段证明了上述观点,如图 10所示,在相同的DOD条件下循环相同圈数后,温度越高,其空隙与NCA正极材料BET表面积的比值越大;在相同的温度条件下,DOD范围越大,其空隙与NCA正极材料BET表面积的比值亦越大.
2.1. 阳离子混排
2.2. 正极表面的化学反应
2.3. 正极材料中过渡金属元素的迁移与溶解析出
2.4. 氧空位
2.5. 高镍正极材料结构相变
2.6. 正极材料微裂纹
-
石墨负极是一种在高镍三元锂离子电池中常用的负极材料,其本身是一种平面层状结构物质,其6个碳原子在平面间以正六边形排列,且每个碳原子与另外3个碳原子相连. 由电子结构排布可知,同平面上的碳原子各有1个p轨道,相互重叠,形成离域π键,电子可在晶格中自由移动. 石墨的晶体结构属于六方晶系,层间以范德华力为结合力,其层间距为0.336 nm,因其具有结合力小、层间距较大的特点,故而层间易滑动. 上述两种结构特征表明,石墨负极层状结构非常有利于锂离子在充放电时进行二维的嵌入和脱嵌. 但在实际充放电过程中,除了锂的嵌入和脱嵌外,锂离子常会与电解液发生溶剂化作用,溶剂化的锂离子容易嵌入到石墨层中,从而影响到正常锂离子嵌脱反应进行,由于溶剂化锂离子嵌入物尺寸产生较大变化,很大程度上会导致石墨自身层状结构的破坏[47],直接影响电极的循环性能,从而导致全电池的容量快速衰减. Wu等人[48]通过对石墨进行卤化处理,使其表面形成较为稳定的C-F结构,有效地防止石墨层状结构的破坏. 天然石墨负极的首次充电容量为351 mAh/g,首周循环过后其不可逆容量可达15.7%,至50次循环后,其电池容量仅为最初的66%. 而卤化处理过后的石墨,其首次充电容量为422 mAh/g,首周循环过后其不可逆容量小于11%,至50次循环后,其电池容量仅降低了7%;卤化处理可提高首周充、放电容量,减少电池的容量衰减.
随着对石墨负极材料研究的深入,发现更换黏结剂可提高结构稳定性,从而提升库伦效率和循环稳定性. Shah等人[49]以聚丙烯腈-丁二烯(NBR)对比常用的商业锂离子电池黏结剂偏氯乙烯(PVDF),发现聚丙烯腈-丁二烯可有效钝化石墨表面,显著提高电极和电解质的相容性. 同时,PVDF的强结合强度将颗粒紧密结合在一起,并使复合材料牢固地结合到集电器上,特别有利于电极的结构稳定性. 与PVDF的聚乙烯基链相比,丙烯腈-丁二烯聚合物链的导电性较高,特别是在高充、放电速率下,有利于Li+电荷传输. 另外,弹性NBR黏合剂的可拉伸特性有助于克服长期循环中石墨的反复体积波动. 因此,选用NBR黏结剂可提高循环稳定性,减少电池的容量衰减.
-
随着研究的深入,人们发现高镍三元正极这类过渡金属的氧化物往往会使电池具有较高的能量密度[50]. 然而,高镍三元锂离子电池存在一些主要问题,当过渡金属从正极材料中溶出,进入到电解液中,极易沉积在负极表面并在负极发生还原反应,从而破坏负极表面的固体电解质界面膜(SEI)(图 11),降低了电池负极的电化学稳定性[51]. 其中,SEI膜是电池进行首次充、放电时,电解液在负极表面发生还原反应沉积在负极表面形成的一层致密钝化膜. 这层钝化膜是一种界面层,具有固体电解质的特征,是电子绝缘体却是Li+的优良导体,Li+可以经过该钝化膜自由地嵌入和脱出,因此这层钝化膜被称为“固体电解质界面膜”. SEI膜具有有机溶剂的不溶性,在有机电解质溶液中能稳定存在,并且溶剂分子不能通过该层钝化膜,从而能有效防止溶剂分子随锂离子发生共嵌入,避免了因溶剂分子共嵌入对负极材料造成的破坏,因而大大提高了电极的循环性能和使用寿命.
在高镍三元正极材料所包含的几种过渡金属中,钴和铝相对稳定,而锰和镍易发生溶解现象[53]. 于锰而言,高氧化态的Mn4+通过接受来自电解液中的电子,会在脱锂过程中在正极表面形成低氧化态的Mn2+和Mn3+,Mn3+会在电解液中发生歧化反应生成Mn2+和Mn4+. 溶解在电解液中的Mn2+迁移到负极,并沉积在负极表面. 王嗣慧等人[54]通过使用XPS谱图,在负极上检测出镍、锰的特征峰信号,并利用ICP-AES这一含量检测方法,具体测量出沉积在负极的过渡金属的含量. Wang等人[55]发现Mn2+溶解进入电解质后会发生溶剂化作用,反应后物质的结构会对溶剂造成严重破坏,如PF6-等,从而使得电池的容量衰减. 张欣等人[56]发现在高温下,正极材料中过渡金属溶解程度会显著增加,使得负极上沉积的过渡金属元素含量增多,加重了容量衰减的程度.
于镍而言,由于低的扩散势垒和晶格中的Ni浓度梯度,Ni极易发生不可逆的还原,使得Ni离子从材料内部迁移到表面并溶解,最后沉积在负极表面,同时促使正极材料表面在充、放电过程中发生结构重构层生长,倍率和循环性能的持续下降. Kim等人[57]发现二价Ni离子在正极中存在的结构缺陷的演化会显著促进相变,导致负极的SEI层生长不均匀,同时也使多孔的正极侧有树突状锂生成. 通过创建具有外延结构的纳米结构稳定剂,使正极中的镍缺陷被明显抑制,防止了镍的迁移,提高了正极材料的结构稳定性和热稳定性,从而延缓了电池的衰减.
负极材料生成的原有的SEI膜被新沉积的过渡金属破坏后,会消耗更多正极材料产生的活性锂离子,从而生成新的SEI膜. 但由于消耗了额外的活性锂离子,放电时无法完全被利用,减少可用的总活性锂离子量,导致电池的容量衰减. 此外,低氧化态的过渡金属离子Mn2+也会与电解液中的HF反应生成MnF2,增大了电池欧姆内阻,通过使用中和酸性物质的溶液可缓解这一问题. 另外,在过渡金属溶出的同时,之前已嵌入到正极材料中的锂离子会被迫脱嵌进入电解液中,造成“自放电”过程,使得电池容量受到损失.
-
负极侧活性物质的损失亦是导致容量衰减的另一个主要因素. 正极材料会因锂化过程而体积膨胀产生裂纹,负极同样也会受到机械应力的影响导致活性物质的损失. 一般电流越大,负极电极越易产生裂纹,另一方面,负极电极的体积和电池的荷电状态(SOC)密切相关,故裂纹的产生使得材料体积发生变化,活性物质被迫损失,影响了SOC状态. 而电池处于高SOC时,主要是由负极侧的电极活性物质损失导致的容量衰减.
为了减弱负极侧活性物质损失所造成的容量衰减,一般情况下采用补锂方法. 最初的常用锂粉微球补锂,可以直接加入负极片表面或是通过负极合浆方法添加[58]. Wang等人[59]利用SBR-PVDF组合体系将稳定锂金属粉末(SLMP)添加进石墨负极中,其中SBR作为黏合剂,PVDF的作用是提供离子传输至材料表面的传输通道. 通过电化学测试发现,以负极合浆的方法进行锂粉补锂,首次库伦效率可从90.6%提高至96.2%. Ai等人[60]将浓度分数均为0.5%的SBR和PS溶于二甲苯中制成黏合剂溶液,再将SLMP加入其中制成稳定的SLMP浆料,均匀涂布在负极表面. 在NCM-石墨组成的全电池体系下进行电化学测试,发现首次库伦效率从82.35%提高至87.8%. 同时,循环稳定性也所有提升. 虽能在一定程度上达到补锂效果,但补锂后存在的电芯安全性问题不容小觑. 张琦等人[61]发明了一种通过在负极两面辊压锂片的补锂方法,以质量比为97.2%的LiNi0.8Co0.1Al0.1O2高镍三元材料为正极,碳纳米管(质量比为0.5%)和碳纤维(质量比为0.3%)混合物为导电剂,质量比为2%的聚偏氯乙烯为黏结剂,将含二氧化硅的硅碳负极两面辊压锂片,制成锂离子电池,通过电化学测试发现其电池能量密度可达到300 Wh/kg,相较于未进行补锂操作的高镍三元正极材料锂离子电池,能量密度提高了20%.
-
除石墨外,硅基负极材料因其比容量高(4 200 mAh/g)、环境友好、成本低等优点,也被添加用作高镍三元正极材料锂离子电池的负极材料[62]. 在充、放电过程中,锂离子嵌入、脱嵌的过程对石墨材料而言,所造成的体积变化较小,结构相对稳定. 但对于硅基负极材料而言,在电池工作中,硅锂结合形成的合金相会造成硅体积的剧烈膨胀[63],其膨胀程度可达300%,导致材料出现粉化现象,从而使锂离子随着电极材料一同流失,导致电池容量衰减. 伴随着硅体积的反复变化,在循环中负极的SEI层不断的破裂、重构,导致锂离子和电解液的持续消耗,加快了电池的容量损失. Lee等人[64]通过在硅基中掺杂钛原子的方法,发现硅在锂化过程中所造成的膨胀会得到有效抑制. 不仅其厚度减小幅度明显,而且在拉曼光谱的测量下发现钛硅材料脱锂后结构相对完整,在2A/g下进行电化学测试,循环300周后仍有较高的稳定容量.
另外,硅与锂合金化后易于电极脱锂电子接触而失活也是硅基作为负极材料导致全电池容量衰减的一大因素. Zhu等人[65]在硅基负极材料中掺杂锗元素,通过形成硅—锗合金的方式来提升锂离子的扩散动力学性能,降低硅对锂离子的捕获率,减少活性锂的不可逆损失,从而达到提升库伦效率的目的.
结合石墨和硅基材料的各自特性,制得硅—碳复合负极材料是提升硅负极循环性能的主要途径之一. Ma等人[66]利用热氧化法制得内部有大量孔隙的石墨,将硅以气相沉积法沉积在石墨上. 硅的体积膨胀受到石墨孔隙的限制,经过100次循环后其膨胀率降低,容量衰减得到缓解,有效地延长了负极的使用寿命.
3.1. 负极自身结构被破坏
3.2. 负极表面SEI膜破坏机制
3.3. 负极材料活性物质损失
3.4. 硅基负极材料衰减机制
-
电解液作为构成电池的重要组成部分,对电池的容量、循环性能、倍率性能等有着很大的影响. 电解液需要满足如下要求:不与电池中的其他组分(正极、负极材料)反应;具有高离子电导率和低电子电导率;不易受温度影响而发生变质;低可燃性;低黏度、高介电常数等. 高镍三元正极材料锂离子电池的电解液锂盐溶质主要有六氟磷酸锂、四氟硼酸锂等. 其中,六氟磷酸锂因其电化学综合性能最佳,是最为常见的一种电解液.
首先,电解液和正、负电极材料会进行直接接触,易发生副反应消耗电极材料和电解液. Wang等人[67]在NCM523正极材料外包覆快速锂离子导体Li2O-B2O3-LiBr,它作为保护壳可阻止酸性电解液和电极的腐蚀反应. Wu等人[68]利用CeO2涂层包覆技术对LiCo1/3Ni1/3Mn1/3O2进行改性,在室温下,含质量分数为1%CeO2涂层材料的正极材料在3C条件下循环12次,容量保留率从86.6%提高到93.2%. 同时利用ICP-OES可证明该涂层材料可以保护过渡金属的溶解,通过EIS分析可知循环性能提高归因于涂层材料有效抑制了电解液和电极之间的反应. Lei等人[69]采用一种表面臭氧水解的方法使Li的成核位点均质化,并指导Li金属在柔性富氧木质素衍生碳膜上均匀沉积. 通过此法,以NCM811为正极材料的金属锂电池在循环230周后库伦效率仍可保持在98%以上,循环时间长达1 000 h以上,实现了提高电池周期寿命且可逆容量高的目标.
其次,高镍三元锂离子电池在充电时,高脱锂态形成了高反应活性的Ni4+易与电解液发生反应,生成较厚的正极固体电解质界面,增大了表面阻抗,阻碍了锂离子的扩散,导致电池的功率、容量衰减,库伦效率降低,还可能伴有析出气体等问题[70]. 但正极固体电解质界面可在一定程度下抑制电极与电解液的反应,因此可以通过设计调控正极固体电解质界面来提高界面稳定性.
再者,电解液易受高工作电压的影响而发生分解,从而导致电池的容量衰减. 以碳酸酯基电解液为例,该电解液会在高镍正极侧容易被氧化,在负极上容易被还原,从而同时会沉积在正、负极表面. 这一沉积层会增加电极界面欧姆内阻,沉积过程产生的气体易导致电池膨胀,并促使HF等强腐蚀性副产物生成[71]. 因此,开发新的电解液或功能添加剂,使其在高压循环下获得正/负极材料与电解质的高相容性尤为迫切. 目前,已开发出用于高压电解液的新溶剂,包括氟化碳酸酯、砜类、腈类等,但商业化应用仍有待进一步验证.
-
高镍三元锂离子电池的电化学性能与正极、负极和电解液息息相关. 本研究系统总结了正极材料会因发生阳离子混排、表面反应、过渡金属元素的迁移与溶解析出、氧空位、相变和微裂纹而使高镍三元锂离子电池电化学性能发生衰减;负极材料的自身结构和表面的SEI膜被破坏、负极材料体积膨胀和捕获锂离子行为也关乎高镍三元电池的容量衰减过程;作为与正、负极相接触的电解液,电解液与两电极的反应和自身的稳定性能亦是影响电池容量衰减的重要因素. 本研究希望能为高镍三元锂离子电池的衰减机制更加深入的研究提供理论支撑,为高镍三元正极材料、石墨和硅负极材料以及电解液的开发提供科学思路:(1) 高镍正极材料应注重材料的结构设计,稳定材料的结构,抑制材料结构转变和微裂缝的形成;(2) 高镍正极包覆、掺杂等改性方法有利于减少材料循环过程中过渡金属的迁移与溶出以及与电解液的副反应;(3) 高容量稳定硅基负极材料的开发将有利于提升电池循环寿命;(4) 面向未来电池固态化,高镍正极固体电解质界面兼容性研究有待加强. 综上最终促进高性能高镍三元锂离子电池的开发.




 下载:
下载: