-
开放科学(资源服务)标志码(OSID):

-
核桃分心木被乡间百姓称作胡桃衣,又叫胡桃隔,是一种干燥木质隔膜,位于核桃果仁之间,富含多酚物质[1]. 多酚不仅具有很强的抑菌、抗衰老、抗辐射等生理功效,还能有效减少氧化应激和抑制大分子氧化[2],已成为食品、医药、染色等领域研究的热点课题之一[3],具有较高的经济价值. 我国核桃种植面积居世界首位,然而核桃分心木却未能得到充分的开发利用. 长期以来,核桃分心木作为废弃物丢弃,不仅造成极大的环境污染,更是资源的严重浪费,因此,若能提取核桃分心木中的多酚物质,研究其抗氧化活性,进一步将其应用于食品防腐保鲜等领域,对核桃分心木的资源化利用具有重要意义[4-5]. 多酚具有酚羟基,根据相似相溶原理,在极性大的溶剂中溶解性较强,比如水、乙醇、丙酮,可溶于乙酸乙酯,不溶于乙醚、氯仿、石油醚等[6-7]. 目前多酚提取技术主要集中在有机溶剂、离子沉淀、树脂吸附分离、超临界二氧化碳萃取、热水浸提等方法[8-9]. 利用这些技术进行提取,存在时间长、能耗大、价格昂贵等诸多问题. 超声处理不仅可缩短提取时间,还能减少能量浪费. 超声辅助提取的机理涉及空化作用[10],超声在溶液中产生空化泡,空化泡的迅速产生和破裂会产生强有力的冲击波和二次效应[11-12],如局部高温高压等,从而对周围植物组织和细胞产生破坏[13],可加速溶剂和细胞内溶液的对流和溶质的交换,提高提取效率[14].
本研究拟采用超声辅助提取法从核桃分心木中提取多酚,利用超声过程中产生的机械、空化效应强化植物多酚提取效果,提高多酚的稳定性;考察料液比、处理时间、乙醇浓度、处理温度、超声功率对多酚提取效果的影响;采用多元二次回归方程优化辅以超声处理提取核桃分心木中多酚物质的工艺条件,以常见的自由基-超氧阴离子、羟基自由基为评价指标,研究分心木多酚对其清除作用;分析核桃分心木中多酚的种类及质量分数,为建立测定核桃分心木中多酚单体的高效液相色谱(HPLC)检测方法提供参考依据[15].
全文HTML
-
核桃青果:以泡核桃30%青果皮颜色由绿变黄或略开裂,坚果发育到固有形状达到采收成熟度时即为果实成熟期,采收地点为贵州省息烽县西山镇猪场村.
福林酚(分析纯),上海源叶生物科技有限公司;无水乙醇、甲醇(分析纯),天津市富宇精细化工有限公司;浓盐酸(分析纯),重庆川东化工集团有限公司;无水碳酸钠(分析纯),天津市永大化学试剂有限公司;BHT(分析纯),上海阿拉丁生化科技股份有限公司;L(+)-抗坏血酸、三(羟甲基)氨基甲烷、焦性没食子酸、水杨酸(分析纯),天津市科密欧化学试剂有限公司;30%过氧化氢、硫酸亚铁(分析纯),成都金山化学试剂有限公司.
-
MS104TS电子天平,梅特勒-托利多仪器上海有限公司;L5S紫外可见分光光度计,上海仪电分析仪器有限公司;3-18R台式高速冷冻离心机,湖南可成仪器设备有限公司;LGJ-22系列冷冻干燥机,四环福瑞科仪科技发展北京有限公司;RE-52AA旋转蒸发器,上海亚荣生化仪器厂;EM-30台式扫描电镜,COXEM;ETD-900M小型离子溅射仪,北京意力博通技术发展有限公司;U3000高效液相色谱仪,赛默飞公司.
-
核桃青果采收后脱去青皮于45 ℃烘箱中烘干,而后破壳取出分心木粉碎过60目筛备用.
-
以乙醇-水溶液作为提取剂,分别选取料液比(1∶20,1∶40,1∶60,1∶80,1∶100 g/mL)、处理时间(10,20,30,40,50 min)、乙醇浓度(10%,30%,50%,70%,90%)、处理温度(20,30,40,50,60 ℃)、超声功率(200,250,300,350,400W),以多酚得率为指标,研究各因素对多酚提取效果的影响.
-
没食子酸标准品溶液:精密称取0.01 g没食子酸标准品,于100 mL容量瓶中用蒸馏水溶解并定容至刻度、摇匀,得100 μg/mL没食子酸对照品储备液(临用现配)[16].
采用Folin-Ciocaileu比色法[16],分别准确移取1.0,2.0,3.0,4.0,5.0 mL没食子酸对照品储备液于10 mL容量瓶中,用蒸馏水定容、摇匀,得浓度为10,20,30,40,50 μg/mL没食子酸溶液[16]. 分别吸取没食子酸溶液1 mL于10 mL容量瓶中,加入5 mL 10%福林酚试剂,摇匀、避光,5 min后加入4 mL 7.5%碳酸钠溶液,摇匀后避光反应1 h,在765 nm处测定吸光度值[16].
-
参考梁杏[16]的测定方法,略有改动,称取粉碎过60目筛的分心木粉末0.2 g置于50 mL离心管中,按料液比1∶60 g/mL加入提取液,置于浴槽式数控超声波清洗器中,超声功率200 W,30 ℃提取20 min,取出,8 000 r/min离心5 min,上清液为提取液. 吸取1 mL提取液放入试管中加入蒸馏水进行一定比例稀释后再吸取1 mL稀释液,根据1.3.3的标准曲线方法测吸光度值,计算多酚得率.
式中,X为样品中多酚得率(mg/g),c为样品多酚浓度(μg/mL),V为提取液总体积(mL),N为稀释倍数,m为样品质量(g).
-
在各影响因素试验的基础上,选取料液比、处理时间、乙醇浓度、处理温度4个因素,以多酚得率为指标,运用多元二次回归方程模型进行试验设计,设计方案见表 1.
-
分心木多酚制备:称取一定质量分心木样品,按料液比1∶80 g/mL加入48%乙醇水溶液,置于浴槽式数控超声波清洗器中,超声功率300 W,61 ℃下提取21 min. 多酚提取液离心后,将上清液在79 ℃下进行减压浓缩,而后进行低温冷冻干燥,制成分心木粗多酚样品.
清除自由基试验:准确称取分心木多酚样品、抗坏血酸(Vc)及2,6-二叔丁基对甲酚(BHT)各0.25 g,用48%乙醇溶解定容至50 mL配成5 mg/mL溶液备用.
-
参考杨喆等[17]的测定方法,略有改动,向具塞试管中分别加入0.05 mol /L pH值为8.2的Tris-HCl缓冲液2.3 mL,浓度为0.01,0.03,0.05,0.07,0.09,0.11 mg/mL的多酚待测液1.0 mL,5 mmol /L邻苯三酚溶液1 mL,混合均匀于室温下准确反应20 min后立即加入2滴浓盐酸终止反应,在420 nm处测定吸光度值(A). 以相同浓度的Vc和BHT溶液代替多酚待测液,作为阳性对照. 计算公式为
式中,C超氧阴离子为超氧阴离子清除率(%),A为多酚待测液吸光度值,A0为以超纯水代替多酚样液并测定空白吸光度值,A1为以超纯水代替邻苯三酚溶液并测定本底吸光度值.
-
采用水杨酸法,取0.1,0.2,0.3,0.4,0.5,0.6 mg/mL核桃分心木多酚样液1.5 mL,依次加入6 mmol/L FeSO4溶液2 mL,6 mmol/L水杨酸-乙醇溶液2 mL,6 mmol/L H2O2溶液2 mL,37 ℃水浴反应30 min后,在波长510 nm处测定吸光度值(A),以相同浓度的Vc和BHT溶液代替多酚待测液,作为阳性对照[18]. 计算公式为
式中,C羟基自由基为羟基自由基清除率(%),A2为以超纯水代替H2O2溶液并测定本底吸光度值.
-
取分心木粉末3.0 g,置于250 mL锥形瓶中加入100 mL 70%的乙醇水溶液,置于浴槽式数控超声波清洗器中,于50 ℃,300 W下,提取20 min,重复提取2次,合并提取液. 提取液经8 000 r/min离心10 min,上清液在79 ℃下减压浓缩至膏状后用5 mL 70%乙醇定容至5 mL,即为多酚提取液,置-4 ℃冰箱中冷藏备用[19](可保存7 d);多酚提取液用0.22 μm亲水滤膜过滤,置于1.5 mL进样瓶中.
-
CAPCELL PAK C18色谱柱,流动相为0.8%乙酸溶液(A相)、色谱甲醇(B相),进样量5 μL,流速0.9 mL/min. 对照品:没食子酸、儿茶素、绿原酸、香草酸、咖啡酸、丁香酸、表儿茶素、丁香醛、对香豆素、阿魏酸采用洗脱程序1,芦丁、杨梅素、胡桃醌、槲皮素采用洗脱程序2. 洗脱程序1为柱温30 ℃,波长280 nm(洗脱梯度:0~10 min,10%~20%B;10~30 min,20%~50%B;30~40 min,50%B;40~41 min,50%~100%B;41~50 min,100%B;50~51 min,100%~10%B;51~60 min,10%B);洗脱程序2为柱温25 ℃,波长251 nm(洗脱梯度:0~10 min,10%~40%B;10~25 min,40%~60%B;25~35 min,60%~80%B;35~36 min,80%~100%B;36~46 min,100%B;46~47 min,100%~10%B;47~57 min,10%B).
-
采用SEM对分心木粉末超声前后进行表观形貌观察,在样品台贴上双面胶,在双面胶上均匀放置少量样品,吹去多余样品,喷金处理,再利用SEM进行扫描观察、拍照.
-
采用EXCEL2007进行数据整理,运用ORIGIN9.1进行制图,采用SPSS19.0软件对试验数据进行ANOVA分析.
1.1. 材料与试剂
1.2. 仪器与设备
1.3. 试验方法
1.3.1. 核桃分心木样品制备
1.3.2. 影响因素试验
1.3.3. 没食子酸标准曲线绘制
1.3.4. 多酚得率计算
1.3.5. 多元二次回归方程设计
1.3.6. 分心木多酚体外清除自由基试验
1.3.6.1. 对超氧阴离子清除作用
1.3.6.2. 对羟基自由基清除作用
1.3.7. 分心木多酚物质高效液相色谱分析
1.3.7.1. 样品溶液的制备
1.3.7.2. 检测条件
1.3.8. 微观结构观察
1.3.9. 数据处理
-
由图 1可知,分心木多酚得率随料液比增大呈先增加后降低的趋势;料液比为1∶80 g/mL时,分心木多酚得率达到最高,为72.87±1.02 mg/g;料液比为1∶20 g/mL时,多酚得率最低,为66.29±0.24 mg/g. 最高点较其他梯度多酚得率增加幅度为9.93%(1∶20 g/mL),6.98%(1∶40 g/mL),差异有统计学意义(p<0.05),3.02%(1∶60 g/mL),1.77%(1∶100 g/mL),差异无统计学意义(p>0.05). 这可能是因为增加溶剂,颗粒可扩散空间增大,有效接触面积增加提高传质推动力,料液比继续加大非多酚物质溶出引起多酚得率下降[20]. 过高的溶剂体积会增大后续提纯等试验过程的工作量,故在后续试验中选择料液比1∶80 g/mL进行处理.
-
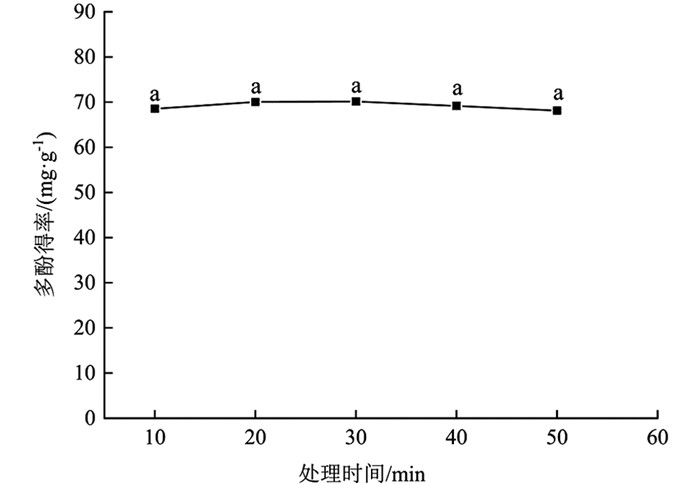
由图 2可知,分心木多酚得率随处理时间延长呈先升高后下降的趋势;10~30 min分心木多酚得率表现为增加,上升幅度为2.25%(10~20 min),0.09%(20~30 min),差异无统计学意义(p>0.05);30~50 min多酚得率逐渐降低,下降幅度为1.37%(30~40 min),1.49%(40~50 min),差异无统计学意义(p>0.05). 30 min时,多酚得率最大,为70.11±0.65 mg/g,较其余时间梯度多酚得率差异无统计学意义(p>0.05). 这说明延长处理时间可增加多酚溶出量,但时间过长,溶剂对物料的作用达到平衡加之氧化原因致使多酚得率下降[21]. 20 min时,多酚得率也较高,为70.05±0.43 mg/g. 考虑到耗能问题,在后续试验中选择20 min进行处理.
-
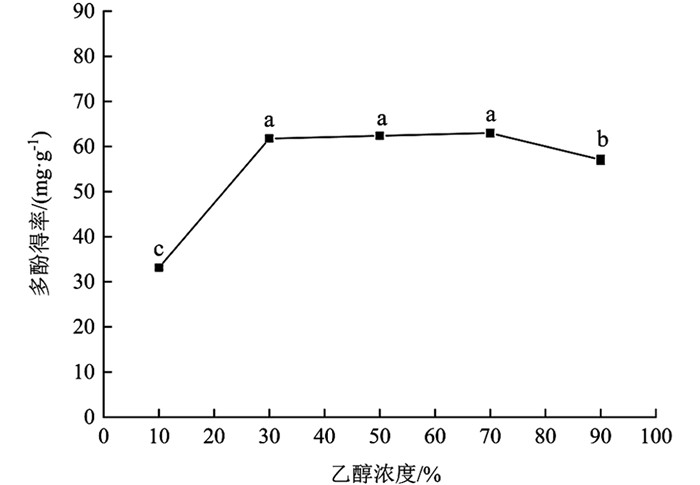
由图 3可知,乙醇浓度在10%~30%范围内时,多酚得率上升速率较快,上升幅度为86.23%,差异有统计学意义(p<0.05);乙醇浓度在30%~70%范围内时,多酚得率上升速率较慢,多酚得率间差异无统计学意义(p>0.05);乙醇浓度在70%~90%范围时,多酚得率降低,差异有统计学意义(p<0.05). 乙醇浓度为70%时,多酚得率最高,为62.98±0.87 mg/g;较其余梯度多酚得率增加幅度为89.81%(10%),10.38%(90%),差异有统计学意义(p<0.05),1.93%(30%),0.93%(50%),差异无统计学意义(p>0.05). 就整体变化趋势而言,分心木多酚得率随乙醇浓度增大逐渐增加而后趋于下降,这可能是提取前期溶剂极性较大,多酚溶出速率较慢,随乙醇浓度逐渐增大,溶剂与多酚极性较为接近,多酚得率增加,但乙醇浓度过高溶剂极性变弱,样品中醇溶性杂质溶出,多酚得率降低[22]. 综合考虑,在后续试验中选择乙醇浓度为50%进行处理.
-
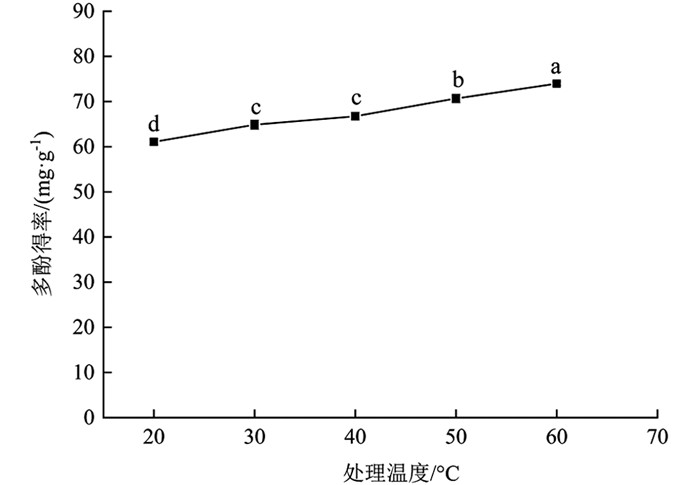
由图 4可知,温度对分心木多酚得率影响有统计学意义(p<0.05). 20~60 ℃时,多酚得率逐渐增加,60 ℃时多酚得率最大,为73.97±0.42 mg/g. 最高点与其余温度梯度相比多酚得率提高21.14%(20 ℃),14.05%(30 ℃),10.80%(40 ℃),4.67%(50 ℃),差异有统计学意义(p<0.05). 提高温度分子运动加快,多酚更易从细胞内部扩散到溶剂中[23]. 考虑到高温对多酚稳定性的影响及能耗问题,在后续试验中选择60 ℃进行处理.
-
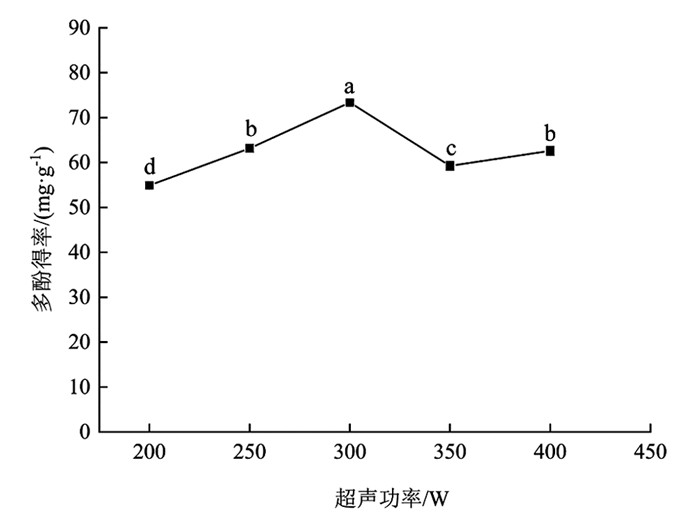
由图 5可知,超声功率对分心木多酚得率影响有统计学意义(p<0.05). 在200~300 W时,多酚得率增加幅度为33.58%,差异有统计学意义(p<0.05);在300~350 W时,多酚得率下降幅度为23.77%,差异有统计学意义(p<0.05);在350~400 W时,多酚得率出现略微上升. 超声功率为300 W时,多酚得率达到最高点,为73.36±0.45 mg/g,较其余梯度多酚得率差异有统计学意义(p<0.05). 可能的原因是超声功率加大,空化作用增强,加速了细胞破碎,多酚得率提高,之后一些极性相同杂质的溶出阻碍了多酚渗出. 超声功率过大,产生较强的声波,介质质点将吸收的声能大部分转变进而生成热能,致使样品组织温度出现升高,部分难溶的酚类物质得以溶出[24]. 综合考虑,在后续试验中选择超声功率为300 W进行处理.
-
在影响因素试验结果的基础上,确定超声功率为300 W,以料液比(X1)、处理时间(X2)、乙醇浓度(X3)和处理温度(X4) 4个因素为自变量,以多酚得率为特征目标物,采用多元二次回归方程模型进行试验,结果见表 2和表 3.
从表 2和表 3可以看出,多元二次回归方程的的失拟值p=0.997 4>0.05. 多元二次回归方程系数R2=0.933 9,说明该模型用于预测核桃分心木多酚得率是可行的;方程模型的变异系数为0.72%,仅有总变异的0.72%不能用该模型进行解释,表明该模型重复性较好;多元二次回归方程的校正系数(0.867 8)与预测系数(0.835 5)较为接近,说明该模型拟合程度较优;信噪比为13.126>4,说明该模型有足够强的信号对分心木多酚得率进行预测,试验误差小. 对该模型进行回归分析可知,X2,X4对分心木多酚得率影响有统计学意义(p<0.05,p<0.01);X1X2的交互作用对多酚得率影响也有统计学意义(p<0.05);X12,X22,X32及X42对多酚得率的影响有统计学意义(p<0.01).
通过多元二次回归方程模型预测分析,得到分心木多酚较佳提取条件为料液比1∶80 g/mL,处理时间21 min,乙醇浓度48%,处理温度61 ℃,理论提取率为75.86 mg/g. 采用理论优化提取条件,验证预测结果的准确性,得到多酚得率平均值为74.72±1.85 mg/g,与理论值相比,相对误差较小,说明该模型对模拟、预测分心木多酚得率可行,提取的技术参数可靠.
-
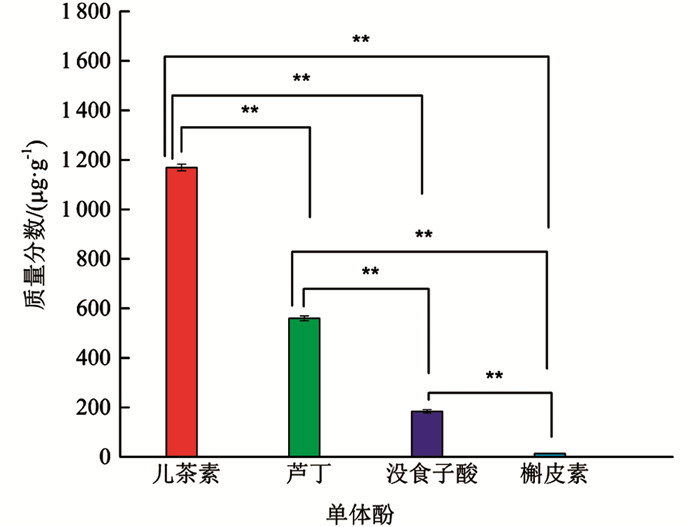
由图 6可知,HPLC从分心木中鉴定出儿茶素、芦丁、没食子酸、槲皮素共4种单体酚,其中儿茶素质量分数最高、芦丁次之、没食子酸较低、槲皮素最低. 儿茶素质量分数较芦丁、没食子酸及槲皮素3种单体酚分别高2.09倍,6.35倍,89.92倍,差异有统计学意义(p<0.01);芦丁与没食子酸、槲皮素相比,质量分数分别高3.04倍,43.08倍,差异有统计学意义(p<0.01);没食子酸较槲皮素而言,质量分数高14.15倍,差异有统计学意义(p<0.01).
-
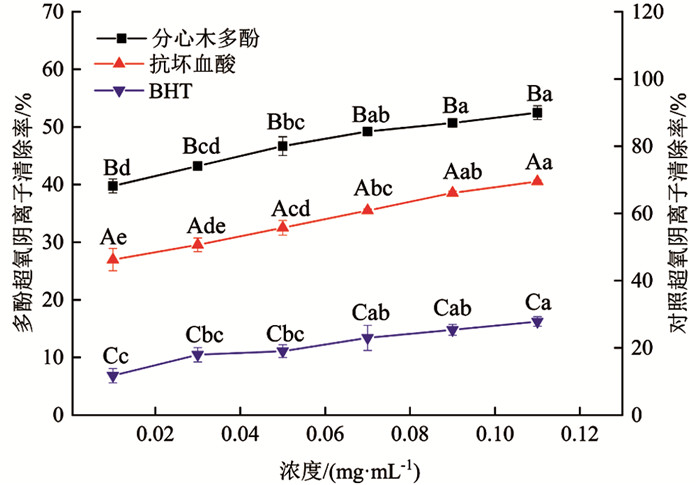
由图 7可知,分心木多酚、抗坏血酸及BHT均对超氧阴离子有较好的清除作用. 清除效果均随浓度增大呈上升趋势,由大到小依次为抗坏血酸、分心木多酚、BHT. 分心木多酚浓度为0.11 mg/mL时,对超氧阴离子清除率达52.47%;抗坏血酸浓度为0.03 mg/mL时,对超氧阴离子清除率达50.64%. BHT在设定浓度范围内对超氧阴离子清除率未达到50.00%;当浓度为0.11 mg/mL时,其BHT清除率最大,也仅为27.82%. 最高点时,抗坏血酸较分心木多酚清除率提高32.44%,分心木多酚较BHT清除率提高88.58%,差异有统计学意义(p<0.05).
-
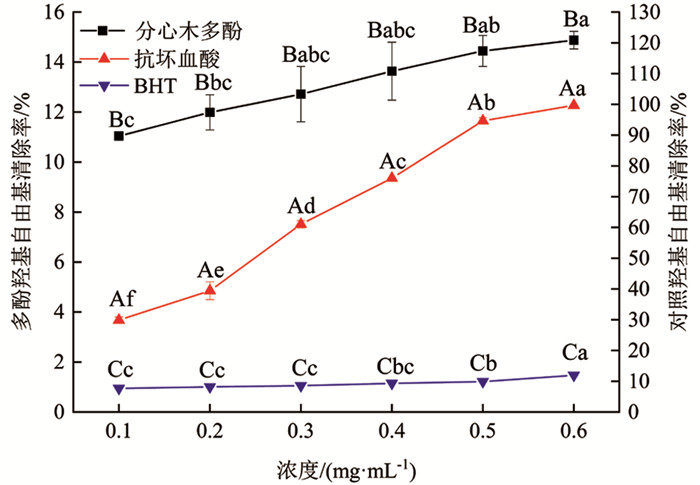
由图 8可知,分心木多酚、抗坏血酸及BHT对羟基自由基均有一定的清除作用. 三者对羟基自由基清除效果均随浓度增大表现为逐渐增加的趋势,由大到小依次为抗坏血酸、分心木多酚、BHT. 分心木多酚和BHT对羟基自由基清除作用均不是很明显,清除率均在20%以下. 分心木多酚和BHT浓度为0.6 mg/mL时,对羟基自由基清除率分别为14.88%和11.93%. 抗坏血酸浓度为0.3 mg/mL时,对羟基自由基清除率已经达到61.04%. 浓度为0.6 mg/mL时,抗坏血酸较分心木多酚羟基自由基清除率提高570.05%,差异有统计学意义(p<0.05);分心木多酚与BHT相比,清除率提高24.73%,差异有统计学意义(p<0.05).
-
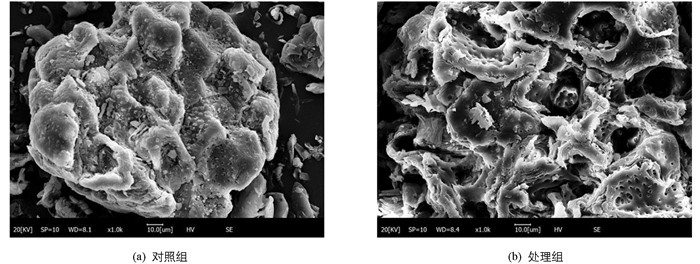
超声处理对分心木粉微观结构影响很大(图 9). 对照组分心木粉呈不规则块状结构,表面较为平整、光滑且有一定的小孔附着其上,结构质地较为紧密;处理组分心木粉形状不规则、组织结构较为松散,呈不规则、凹陷、折叠起皱的球形,表明超声过程中产生的机械、空化及湍流等复合效应能破坏植物组织结构,可将细胞膜网状结构撕裂,导致其结构出现坍塌,有利于多酚物质渗透、扩散进入提取溶液中,强化了植物多酚的提取效果.
2.1. 影响因素试验
2.1.1. 料液比对分心木多酚提取效果的影响
2.1.2. 处理时间对分心木多酚提取效果的影响
2.1.3. 乙醇浓度对分心木多酚提取效果的影响
2.1.4. 处理温度对分心木多酚提取效果的影响
2.1.5. 超声功率对分心木多酚提取效果的影响
2.2. 分心木多酚提取回归模型结果
2.3. 分心木单体酚种类及质量分数
2.4. 分心木多酚体外清除自由基试验结果
2.4.1. 超氧阴离子清除效果
2.4.2. 羟基自由基清除效果
2.5. 超声处理对分心木粉微观结构的影响
-
多酚作为天然的植物次生代谢产物,是能潜在促进健康的一种化合物,因具有多种生物活性功能,是近年核桃副产物研究的主要方向. 本研究基于超声效应并结合多元二次回归方程来解析核桃分心木中多酚物质的响应变化规律和优化多酚提取工艺参数,得出分心木多酚较优提取参数:料液比1∶80 g/mL,处理时间21 min,乙醇浓度48%,处理温度61 ℃,在此条件下多酚得率为74.72±1.85 mg/g. 李瑞等[25]优化云南核桃分心木多酚提取工艺参数为料液比1∶102 g/mL,复合酶添加量0.90%,温度45.33 ℃,多酚得率为22.35 mg/g,研究结果与本研究得出的结论存在差异,究其原因可能是提取方法不同. 李瑞等[25]以酶法为基础进行多酚提取,提取过程中酶解植物细胞壁不彻底,引起多酚溶出较少,导致多酚得率较低. 刘静等[26]优化核桃分心木多酚超声辅助提取工艺参数为料液比1∶62 g/mL,乙醇浓度50%,超声时间50 min,超声温度71 ℃,多酚得率为6.98%. 此外,陈冠林等[27]优化分心木多酚提取工艺,确定多酚提取的较优工艺组合为料液比1∶40 g/mL,乙醇浓度30%,超声时间15 min,提取温度50 ℃,多酚得率为56.46 mg/g,较本研究结果多酚得率偏低,其原因可能是粉碎粒度不同. 本研究粉碎过60目筛,而陈冠林等[27]研究中仅过20目筛,分心木样品颗粒直径较大,提取过程中乙醇溶剂浸提多酚不完全,而且所用乙醇浓度和提取温度偏低,依据相似相溶原理,乙醇和多酚均具有羟基基团,是极性物质,30%乙醇极性较强,与多酚极性相差较大,提取温度偏低,浸提过程中分子运动较弱,扩散较慢,直至浸提结束尚有部分多酚物质未渗入浸提溶剂. 综上,本研究得出的较优工艺与李瑞等[25]相比,处理温度适当,多酚得率增加显著,避免了酶解细胞组织不充分的技术弊端,而且所用料液比较小,利于试验后期开展多酚纯化工作. 与刘静等[26]相比,料液比、乙醇浓度和多酚得率相差不大的情况下,大大缩短了处理时间,节约了提取成本,并且本研究所采用的处理温度较为妥当,避免处理温度过高破坏了多酚物质结构后易引起其氧化. 与陈冠林等[27]相比,虽然采用的提取技术参数均相对较高,但却可以提高分心木多酚得率,具有一定的优越性.
-
自由基是未配对电子,含有1个或多个活性分子,通过破坏蛋白质、核酸及脂质等引发多种疾病,而抗氧化剂能有效清除体内产生的自由基,进而降低氧化应激反应以防止细胞损伤,减轻自由基对人体健康的伤害. 核桃分心木中含有黄酮、酚酸类等化合物赋予其显著的抗氧化能力. 本研究发现超氧阴离子(O2-)、羟基自由基(·OH)均可被分心木多酚清除,羟基自由基清除能力弱于超氧阴离子,这一发现与王新然[18]研究结果一致. 赵娟娟[28]发现分心木黄酮浓度为2 mg/mL时,对O2-和·OH的最大清除率分别为36.90%,66.20%,而本研究得出分心木多酚对O2-和·OH最大清除率分别为52.47%,14.88%. 综上可知,分心木多酚虽具有一定抗氧化能力,然而其抗氧化活性与单体酚化学结构关系密切,羟基数目的多少、羟基位置的变化及羰基结构的差异、糖苷键的不同位置等均会对多酚抗氧化能力产生影响.
-
陈冠林等[27]从核桃分心木中鉴定出14种单体酚,与本研究共有的单体酚为儿茶素、芦丁、没食子酸,本研究中儿茶素质量分数是陈冠林等[27]的4.72倍,而芦丁和没食子酸质量分数均低于陈冠林等[27]的研究结果. 此外,陆胜波等[29]从泡核桃的根,枝,叶,果实(青皮、内种皮、去皮种仁)中共检测到14种多酚物质,与本研究共有的单体酚为儿茶素、芦丁、没食子酸和槲皮素. 本研究中儿茶素质量分数高于陆胜波等[29]的研究结果;芦丁质量分数高于枝条、青皮及去皮种仁,低于根、叶及内种皮且差异较大;没食子酸质量分数低于内种皮,高于其余组织;槲皮素质量分数与去皮种仁接近,但明显低于叶和内种皮. 综上可知,不同泡核桃组织器官单体酚的组成及质量分数是存在差异的,这可能与处理方式、品种差异及地域、气候、海拔等因素有关,多酚物质具有明显的器官特异性.
3.1. 核桃分心木多酚提取工艺
3.2. 核桃分心木多酚抗氧化能力
3.3. 核桃分心木多酚种类
-
通过多元二次回归方程模型对核桃分心木多酚提取工艺进行拟合,得出的优化工艺参数可靠,说明该模型可以较好地模拟和预测核桃分心木中多酚的提取效果. 本研究得出的较佳工艺参数与前人研究相比,在保证多酚得率的基础上节约了提取成本. 基于超声处理过程中产生的各种叠加效应强化了植物多酚的提取效果,在一定程度上克服了部分提取技术的缺陷. 此外,核桃分心木酚类物质鉴定仅用高效液相色谱分析存在一定局限性,后期研究可进行液质联用、气质联用及核磁共振联用质谱等更为先进的技术. 此外还应关注核桃分心木发育过程中多酚物质的合成与积累,从有关基因表达和酶活等分子水平上探索其代谢途径和调控机理. 以细胞氧化应激模型评价天然产物的抗氧化活性是一种低成本且直观反应抗氧化损伤效果的方法. 本研究中分心木多酚对超氧阴离子、羟基自由基均可清除,然而二者的清除效果却有所不同. 丁建英等[20]研究了枇杷叶多酚的抗氧性活性;王鹏等[30]研究了普通枇杷与野生枇杷总黄酮、总酚及抗氧化活性;邹灵秀等[31]研究了白藜芦醇对兔成纤维细胞氧化应激损伤的保护作用. 通过对体外清除自由基和细胞水平的研究,可以在一定程度上反应天然产物的抗氧化效应,从而为后续分子机制的研究提供依据. 后期可开展核桃分心木中单体酚的抗氧化能力及其构效关系的研究. 此外,在细胞研究的基础上再通过动物试验,在日粮中添加一定剂量的天然抗氧化剂来缓解和修复氧化应激造成的伤害,从而为天然抗氧化剂在生产实践中的应用奠定基础.




 下载:
下载: